Imaging CDK4/6 Broaden Options of Breast Cancer Diagnostics with Positron Emission Tomography
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
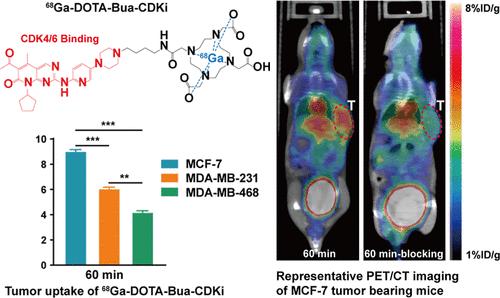
This study developed a novel PET radiotracer to screen breast cancer patients sensitive to CDK4/6 inhibitors, guiding personalized treatment. Two CDK4/6-targeting precursors were synthesized and evaluated in vitro and in vivo. Three breast cancer cell lines─MCF-7, MDA-MB-231, and MDA-MB-468─were selected based on decreasing sensitivity to palbociclib. Compared to [68Ga]Ga-DOTA-Hexa-CDKi, [68Ga]Ga-DOTA-Bua-CDKi clearly identified cell lines with high sensitivity to palbociclib. PET/CT imaging showed significantly higher uptake of [68Ga]Ga-DOTA-Bua-CDKi (8.40 ± 0.85%ID/g) in MCF-7 tumors 60 min after tracer injection, with significant differences in tumor uptake among the three models (P < 0.05). Blocking assays demonstrated specific tumor uptake of [68Ga]Ga-DOTA-Bua-CDKi. Biosafety tests validated its safety as a diagnostic agent. [68Ga]Ga-DOTA-Bua-CDKi showed highly specific targeting of CDK4/6 and effective contrast imaging in tumor models. To our knowledge, [68Ga]Ga-DOTA-Bua-CDKi is one of the first radiotracers to assess CDK inhibitor sensitivity, offering promise for evaluating patient responses to CDK4/6 inhibitors.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


