Identification of constrained peptidomimetics carrying a Michael acceptor warhead as antitrypanosomal agents
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
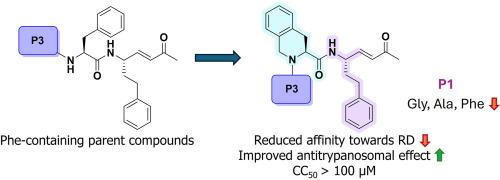
In this structure-activity relationship (SAR) study, we report the development of rhodesain-targeting peptidomimetics with antitrypanosomal activity. The new compounds (SPR65-SPR80) feature the 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (Tic) moiety as conformationally constrained Phe analog. Various substituents were inserted at the P1 and P3 positions, and the methyl vinyl ketone moiety was introduced as warhead. The incorporation of Tic resulted in reduced affinity against rhodesain compared to the parent compounds containing Phe (2a-m), suggesting that its rigidity negatively affects target binding. Nevertheless, promising EC50 values ranging from 0.42 to 1.35 μM were observed in cell-based assays, probably due to better pharmacokinetic properties and/or interactions with additional protozoal targets. CC50 values > 100 μM were observed. Therefore, while Tic is less tolerated by rhodesain, its incorporation in peptidomimetic Michael acceptors led to antitrypanosomal effects that were comparable or slightly better than those of the parent compounds and no cytotoxicity up to 100 μM. These findings could be taken into consideration in future SAR studies aimed at the development of antitrypanosomal agents.

求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


