Design, synthesis, and antitumor activity of NSDs inhibitors targeting lung squamous cell carcinoma
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
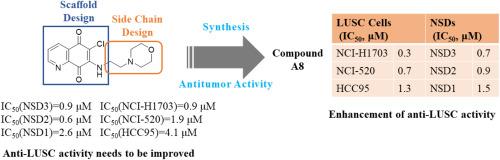
Lung squamous cell carcinoma (LUSC), a highly aggressive subtype of lung cancer, presents significant therapeutic challenges due to its complex molecular underpinnings. Recently, NSD3 has been identified as a key driver in the pathogenesis of LUSC, providing a new direction for targeted interventions. Herein, we report the rational design, synthesis, and comprehensive biological evaluation of a series of NSD3 inhibitors, culminating in the identification of compound A8, which demonstrates potent inhibitory activity against NSD3 and LUSC cell proliferation, with an IC50 of 0.355 μM in NCI–H1703 cells, and less toxicity to non-cancerous HEK293T cells. Cellular thermal shift assays confirmed the binding affinity of compound A8 for NSD3, promoting protein stabilization. Mechanistic investigations revealed that compound A8 induces apoptosis in LUSC cells in a dose-dependent manner and exhibits significant antitumor effects in both in vitro and in vivo models. Notably, compound A8 displayed favorable pharmacokinetic properties and efficaciously suppressed tumor growth in an NCI–H520 xenograft mouse model without observable adverse effects. These findings collectively establish compound A8 as a promising candidate for the development of targeted therapies against LUSC, highlighting the therapeutic potential of NSD3 inhibition.

求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


