Design, synthesis and biological evaluation of pyrrolopyrimidine urea derivatives as novel KRASG12C inhibitors for the treatment of cancer
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
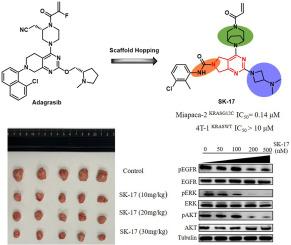
The KRASG12C mutation, which occurs in approximately 14% of lung adenocarcinomas, has recently become a crucial target for therapy via small molecules that covalently bind to the mutated cysteine. In this study, a novel series of pyrrolopyrimidine derivatives was rationally designed and synthesized, employing a structure-based drug design strategy. Through structure-activity relationship (SAR) analysis, compound SK-17 emerged as a direct and highly potent inhibitor of KRASG12C. Cellular assays illustrated that SK-17 exhibits potent antiproliferative effects, induces apoptosis, possesses anti-tumor metastasis properties, and effectively inhibits the downstream KRAS pathway in a dose-dependent manner. Moreover, the synergistic enhancement observed when SK-17 is combined with SHP2 inhibitors in vitro underscores its innovative potential in combinatorial therapies. In the xenograft mouse model, SK-17 demonstrated outstanding tumor growth suppression with good safety. Importantly, the in vivo test results show that compound SK-17 has a superior PK profile and lower toxicity in zebrafish test. These results demonstrated the potential of SK-17 with novel scaffold as a promising lead compound targeting KRASG12C to guide in-depth structural optimization.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


