Using Transient State Kinetics to Contextualize the Catalytic Strategy of Human Ferroptosis Suppressor Protein 1
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
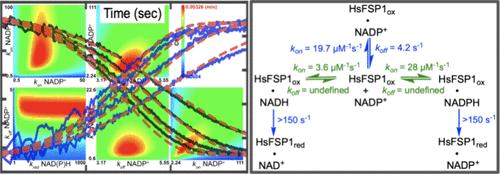
Human ferroptosis suppressor protein 1 (HsFSP1) is an NAD(P)H:quinone oxidoreductase with broad substrate specificity that has been widely implicated in aiding malignant neoplastic cell survival. FSP1 is myristoylated and associated with membranes, where it regenerates the reduced forms of quinones using electrons from NADPH. The quinol products intercept reactive oxygen species and ameliorate lipid peroxidation, preventing ferroptosis, a form of regulated cell death. While FSP1 enzymes have been reported to have 6-OH-FAD as an active cofactor, aerobic titration of the enzyme with NADPH in the presence and absence of ubiquinone (UQ) reveals that this is more likely an artifact and that the native form of HsFSP1 has unmodified FAD as the cofactor. Moreover, HsFSP1 suppresses the reaction of the reduced FAD with molecular oxygen three-fold which, from a kinetic standpoint, severely limits the opportunity for cofactor modification. The isolated form of the enzyme has NADP+ bound and the rate of release of this product limits the observed rate of reduction by NAD(P)H molecules. The reduction of substrate quinones occurs rapidly (≥2000 s–1), dictating that the rate of turnover is wholly defined by the rate of release of NADP+ from the HsFSP1·NADP+ complex. Given that HsFSP1 does not distinguish ubiquinone from ubiquinol by significant differences in binding affinity, this pronounced catalytic commitment to quinone reduction serves to overcome presumed kinetic limitations imposed by the abundance of ubiquinol relative to ubiquinone in the membrane. This characteristic also maintains the enzyme ostensibly fully in the oxidized state under turnover conditions, preventing significant futile reduction of dioxygen.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


