Enhancing Microalgae Cell Inactivation Through Hydrodynamic Cavitation: Insights from Flow Cytometry Analysis
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
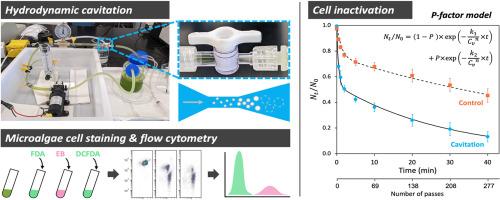
Hydrodynamic cavitation (HC) has recently emerged as an effective method for disrupting microalgae cells with lower energy consumption compared to traditional mechanical methods like bead milling and ultrasonication. However, the efficiency and energy utilization of HC can vary depending on the microalgae species and operating conditions. To assess the efficacy of HC and quantify the extent of cellular damage at the cell level, we designed a bench-top cavitation system to investigate the time-dependent responses of the microalgae Dunaliella viridis to multiple HC passes. We evaluated cell disruption efficiency by monitoring cell concentration using a cell counter and analyzing cell size distribution and whole cell counts via flow cytometry. Additionally, we assessed cell viability, metabolic activity, and reactive oxygen species (ROS) levels using the fluorescent probes fluorescein diacetate (FDA), erythrosine B (EB), and 2′,7′-dichlorofluorescein diacetate (DCFDA). We further analyzed cell chlorophyll autofluorescence and the kinetics of overall cell inactivation. Our results demonstrated that HC effectively disrupted and inactivated cells, approximating pseudo-first-order kinetics. However, the cell inactivation rate and energy utilization efficiency declined rapidly in the early stages, likely due to the accumulation of cell debris. A P-factor model incorporating two first-order rate constants was then developed to better predict the cell inactivation kinetics and further demonstrated that cavitation number alone was insufficient to characterize the dynamic change in the inactivation rate during cavitation. To maintain high inactivation efficiency, it is recommended to keep the cell debris fraction below 10-20%. HC was found to inactivate cells by rupturing cell membranes, leading to the rapid release of intracellular contents such as esterase and chlorophyll. HC did not affect intracellular esterase activity or chlorophyll content in cells with intact membranes, but the endogenous ROS levels in viable cells were reduced. The mechanisms of cell damage were discussed in detail. For bioproduct harvesting, cell membrane integrity is suggested as a key physiological endpoint for optimizing HC treatment protocols.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


