Structural Effects on the Hydride-Tunneling Kinetic Isotope Effects of NADH/NAD+ Model Reactions: Relating to the Donor–Acceptor Distances
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
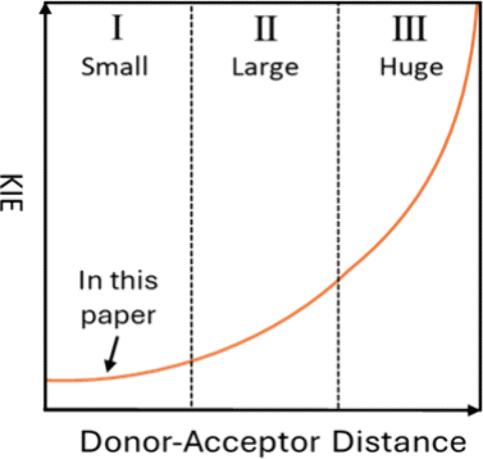
Contemporary H-tunneling theories predict that a longer donor–acceptor distance (DAD) corresponds to a larger kinetic isotope effect (KIE). Herein, hydride-tunneling reactions of NADH/NAD+ analogues in acetonitrile were used to examine the KIE–DAD relationship. Reaction pairs of similar tunneling-ready conformations were selected, so that additional factors influencing KIEs would be relatively fixed. Positive results were obtained, with some reaction pairs displaying a reversal of the traditional KIE−ΔG° relationship in favor of the KIE–DAD relationship, lending strong support to the latter.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


