Challenges in Histological Endpoints for MASH Therapies: An Exercise in Statistical Modelling
IF 6.6
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
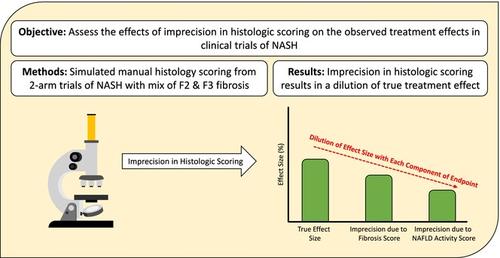
BackgroundRegulatory‐accepted efficacy endpoints for nonalcoholic steatohepatitis (NASH; recently updated to metabolic‐dysfunction associated steatohepatitis, MASH) clinical trials include fibrosis improvement with no worsening of NASH or NASH resolution with no worsening of fibrosis determined by liver biopsy using the NASH Clinical Research Network criteria. These endpoints involve the scoring of four liver histology parameters, all of which are associated with significant inter−/intra‐reader variability. Since few trials have shown positive results with these endpoints, we evaluated the effects of imprecision in histologic scoring on trial results from a statistical perspective.MethodsEstimating the probability (sensitivity) of accurately scoring histology is based on the relationship between measures of agreement and sensitivity. We simulated kappa values for a range of sensitivities. Then, using published kappa values from NASH trials, we selected corresponding sensitivities for histology parameters. Finally, simulations assuming a range of “overscore” and “underscore” probabilities were conducted to estimate the dilution of the true effect size.ResultsSimulations for 2‐arm trials with sample sizes of 400 (mix of stage 2/3 fibrosis) subjects showed ~50% dilution of the true effect size for both approvable endpoints due to scoring imprecision. Such dilution remains constant regardless of sample size.ConclusionImprecise histologic scoring disproportionately impacts the ‘superior’ arm as the error is proportional to the true response rate. This dilution of effect size should be considered when weighing the clinical benefit and the overall risk–benefit profile in the review of NASH studies. This argues for the adoption of non‐invasive biomarkers rather than histologic endpoints.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
15.60
自引率
7.90%
发文量
527
审稿时长
3-6 weeks
期刊介绍:
Alimentary Pharmacology & Therapeutics is a global pharmacology journal focused on the impact of drugs on the human gastrointestinal and hepato-biliary systems. It covers a diverse range of topics, often with immediate clinical relevance to its readership.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


