Enantioconvergent nucleophilic substitution via synergistic phase-transfer catalysis
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
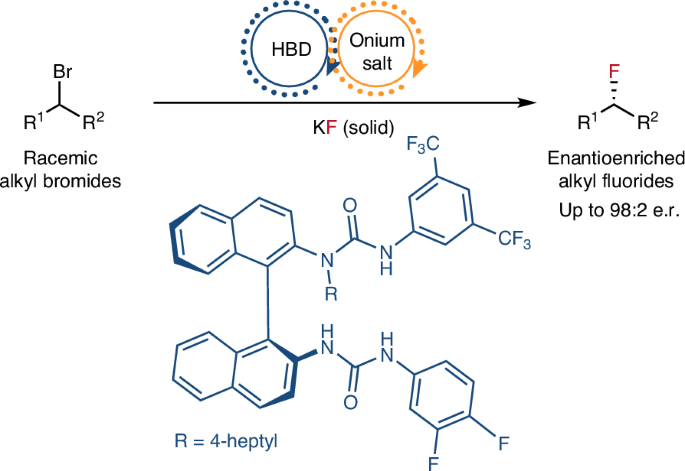
Catalytic enantioconvergent nucleophilic substitution reactions of alkyl halides are highly valuable transformations, but they are notoriously difficult to implement. Specifically, nucleophilic fluorination is a renowned challenge, especially when inexpensive alkali metal fluorides are used as fluorinating reagents due to their low solubility, high hygroscopicity and Brønsted basicity. Here we report a solution by developing the concept of synergistic hydrogen bonding phase-transfer catalysis. Key to our strategy is the combination of a chiral bis-urea hydrogen bond donor (HBD) and an onium salt—two phase-transfer catalysts essential for the solubilization of potassium fluoride—as a well-characterized ternary HBD–onium fluoride complex. Mechanistic investigations indicate that this chiral ternary complex is capable of enantiodiscrimination of racemic benzylic bromides and α-bromoketones, and upon fluoride delivery affords fluorinated products in high yields and enantioselectivities. This work provides a foundation for enantioconvergent fluorination chemistry enabled through the combination of a HBD catalyst with a co-catalyst specifically curated to meet the requirement of the electrophile. The catalytic enantioconvergent nucleophilic fluorination of alkyl halides using inexpensive alkali metal fluorides is a persistent challenge. Now this has been achieved by synergistic hydrogen bonding phase-transfer catalysis combining a chiral bis-urea hydrogen bond donor and an onium salt.

通过协同相转移催化的对映收敛亲核取代
烷基卤化物的催化对映收敛亲核取代反应是非常有价值的转化,但它们是出了名的难以实现。具体来说,亲核氟化是一个众所周知的挑战,特别是当廉价的碱金属氟化物由于其低溶解度、高吸湿性和Brønsted碱度而被用作氟化试剂时。在这里,我们通过发展协同氢键相转移催化的概念报告了一个解决方案。我们策略的关键是将手性双尿素氢键供体(HBD)和铵盐(对氟化钾的增溶至关重要的两种相转移催化剂)结合起来,形成表征良好的三元HBD -氟化铵配合物。机理研究表明,该手性三元配合物对外消旋苯溴化物和α-溴酮具有对映辨别能力,并在氟化后产生高收率和对映选择性的氟化产物。这项工作为通过HBD催化剂与专门为满足亲电试剂要求而设计的助催化剂的组合实现对映会聚氟化化学提供了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


