Structure and mechanism of haem-dependent nitrogen–nitrogen bond formation in piperazate synthase
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
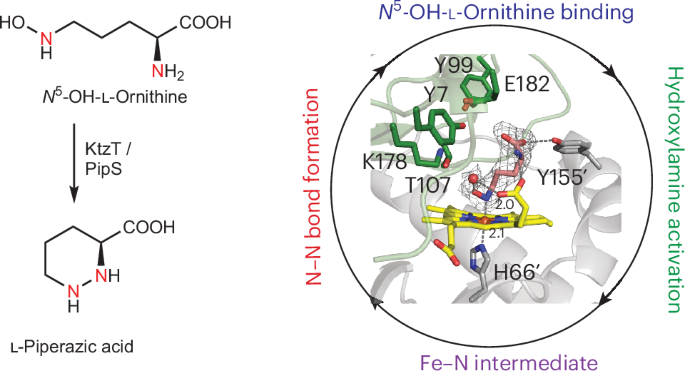
Molecules with nitrogen–nitrogen (N–N) bonds include diverse specialized metabolites from nature, but little is known about the underlying enzymatic mechanisms that have evolved for N–N bond formation. To directly form a single N(sp3)–N(sp3) bond, enzymes must reverse the typical nucleophilicity of one nitrogen. Here we report the structure of PipS, a haem-dependent enzyme that catalyses N–N bond formation in the cyclization of N5-OH-l-ornithine, giving l-piperazic acid. Our work reveals the role of a Lys–Thr dyad early in the mechanism and shows that PipS catalyses either N–N bond formation or imine-group formation in a substrate-specific manner, which may stem from a shared nitrenoid intermediate that effectively reverses the nucleophilicity of the hydroxylamine nitrogen. Our work expands knowledge of enzymatic N–N bond formation and delineates the catalytic versatility of a haem cofactor, paving the way for genetically encoded biocatalysts for N–N bond formation. Despite the existence of many N–N-containing natural metabolites, little is known about the enzymatic mechanisms of N–N bond formation. Now, a catalytically relevant X-ray crystal structure of an N–N-bond-forming enzyme, PipS, is reported and detailed insights into its catalytic mechanism are provided.

哌酸合酶中血依赖氮-氮键形成的结构和机制
具有氮-氮(N-N)键的分子包括来自自然界的多种特殊代谢物,但对进化成N-N键的潜在酶机制知之甚少。为了直接形成单个N(sp3) -N (sp3)键,酶必须逆转一个氮的典型亲核性。在这里,我们报道了PipS的结构,PipS是一种血液依赖性酶,在n5 - oh -l-鸟氨酸环化过程中催化N-N键形成,得到l-哌酸。我们的工作揭示了Lys-Thr二偶体在早期机制中的作用,并表明PipS以一种底物特异性的方式催化N-N键形成或亚胺基形成,这可能源于一种共享的类氮中间体,该中间体有效地逆转了羟胺氮的亲核性。我们的工作扩展了酶促N-N键形成的知识,并描绘了血红素辅助因子的催化多功能性,为遗传编码的N-N键形成生物催化剂铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


