Redox-Induced Engineering of Amorphous/Crystalline MnFeOx Catalyst Enables H2O/SO2-Tolerant NOx Abatement at Ultra-Low Temperatures
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
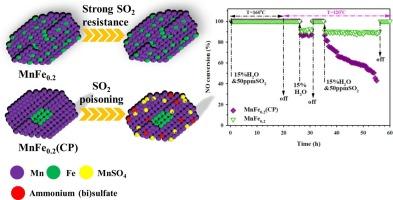
Enhancing resistance to H2O and SO2 poisoning below 150 °C is essential for advancing Mn-based oxide catalysts in ultra-low temperature NH3-SCR of NO. To address this challenge, an amorphous/crystalline MnFey catalyst with engineered Mn-O-Fe interfaces and abundant surface defects was developed using a redox-induced precipitation method. The optimized MnFe0.2 catalyst demonstrates exceptional catalytic performance, achieving over 90% NO conversion and N2 selectivity across a broad 120–260 °C range under highly humid conditions (15 vol% H2O). Most significantly, MnFe0.2 maintains remarkable stability under high humidity and SO2 at 120 °C for 60 h, vastly outperforming conventionally coprecipitated MnFe0.2(CP), which gradually deactivates. This superior performance is attributed to the uniform elemental distribution in MnFe0.2, which enhances the Mn-O-Fe redox cycle through improved electron transfer. These features promote superior low-temperature reducibility and acidity, enabling effective reactant adsorption and activation. Mechanistic studies further reveal that SO2 exposure deactivates MnFe0.2(CP) by forming ammonium (bi)sulfates and MnSO4, which hinder reactant adsorption and subsequent reactions. In contrast, the engineered Mn-O-Fe interfaces in MnFe0.2 enable Fe species to preferentially interact with SO2, shielding Mn from sulfation and significantly reducing deactivation. This work demonstrates a significant breakthrough in catalyst design for ultra-low temperature NH3-SCR, paving the way for the broader application of Mn-based catalysts in industrial NOx control technologies.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


