Electrochemical decarboxylative oxidation of carboxylic acid mixtures on boron doped diamond electrodes
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
Electrochemical decarboxylation of carboxylic acids is of emerging interest for upgrading of pyrolysis oil. In this study, electrooxidation of propionic acid using BDD electrodes in batch mode is shown to form ethanol and ethylene. The influence of process conditions is elaborated on, and we generally demonstrate that the oxidation of propionic acid on BDD is largely induced by solution based hydroxyl radical chemistry. A high relative ethanol over ethylene Faradaic Efficiency (FEethanol/ethylene) can be achieved at low current density (25 mA/cm2) and an electrolyte pH of 5, while the FEethanol/ethylene is affected by consecutive oxidation of ethanol to acetaldehyde and/or CO2. This is further corroborated by investigating the specificity of BDD in electrooxidation of short chain carboxylic acid mixtures. It is shown that the oxidation specificity is not solely dependent on the rate constant of Formic, Acetic, and Propionic acid with hydroxy radicals, but that surface mediated reactions are also relevant. Finally, we propose mitigation strategies for consecutive oxidation of ethanol at high current densities (100 mA/cm2), using flow cells and pulsed electrolysis to control the near-surface concentration of hydroxyl radicals. In conclusion, this study provides a comprehensive analysis of decarboxylation reactions using BDD anodes and additionally emphasizes the significance of engineering approaches to achieve substrate specificity, a field that has hitherto remained largely unexplored.

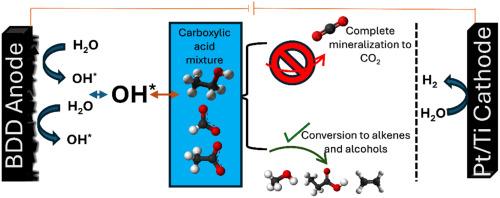
掺杂硼金刚石电极上羧酸混合物的电化学脱羧氧化
羧酸的电化学脱羧是热解油升级的新研究方向。在本研究中,使用BDD电极在间歇模式下对丙酸进行电氧化,生成乙醇和乙烯。对工艺条件的影响进行了阐述,普遍认为丙酸在BDD上的氧化主要是由溶液基羟基自由基化学引起的。在低电流密度(25 mA/cm2)和电解质pH值为5的情况下,可以实现较高的相对乙醇对乙烯的法拉第效率(fe乙醇/乙烯),而乙醇/乙烯受到连续氧化为乙醛和/或CO2的影响。通过研究BDD在短链羧酸混合物电氧化中的特异性,进一步证实了这一点。结果表明,氧化特异性不仅取决于甲酸、乙酸和丙酸与羟基自由基的反应速率常数,而且与表面介导的反应也有关。最后,我们提出了缓解乙醇在高电流密度(100 mA/cm2)下连续氧化的策略,使用流动电池和脉冲电解来控制羟基自由基的近表面浓度。总之,本研究提供了使用BDD阳极的脱羧反应的全面分析,并强调了工程方法实现底物特异性的重要性,这一领域迄今为止仍未得到很大程度的探索。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


