Balanced plant helper NLR activation by a modified host protein complex
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
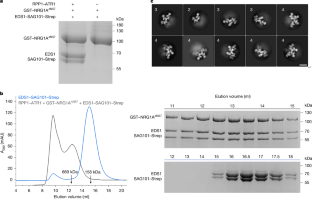
Nucleotide-binding leucine-rich repeat (NLR) receptors play crucial roles in plant immunity by sensing pathogen effectors1. In Arabidopsis, certain sensor NLRs function as NADases to catalyse the production of second messengers2,3, which can be recognized by enhanced disease susceptibility 1 (EDS1) with its partner senescence-associated gene 101 (SAG101), to activate helper NLR N requirement gene 1 (NRG1)4. A cryoelectron microscopy structure shows that second-messenger-activated EDS1–SAG101 mainly contacts the leucine-rich repeat domain of NRG1A to mediate the formation of an induced EDS1–SAG101–NRG1A complex. Structural comparisons show that binding of a second messenger induces conformational changes in EDS1–SAG101, which are recognized by NRG1A, leading to its allosteric activation. We further show that an inhibitory NRG1 family member, NRG1C, efficiently outcompetes NRG1A for binding to second-messenger-activated EDS1–SAG101. These findings uncover mechanisms for NRG1A activation through its recognition of a modified host EDS1–SAG101 complex, and NRG1A inhibition by NRG1C through sequestration of the activated EDS1–SAG101, thus shedding light on the activation and constraint of a central plant immune response system. In Arabidopsis, mechanisms for NRG1A activation by recognition of a modified host EDS1–SAG101 complex, and NRG1A inhibition by NRG1C through sequestration of activated EDS1–SAG101, show activation and constraint of a central plant immune response system.

通过修饰的宿主蛋白复合物平衡植物辅助NLR的激活
核苷酸结合的富亮氨酸重复序列(NLR)受体通过感应病原体效应在植物免疫中发挥重要作用。在拟南芥中,某些NLR传感器作为nadase催化第二信使的产生,这可以通过增强的疾病易感性1 (EDS1)及其伴侣衰老相关基因101 (SAG101)来识别,从而激活辅助NLR N需求基因1 (NRG1)4。低温电镜结构显示,第二信使激活的EDS1-SAG101主要与NRG1A富含亮氨酸的重复结构域接触,介导诱导EDS1-SAG101 - NRG1A复合物的形成。结构比较表明,第二信使的结合诱导EDS1-SAG101的构象变化,这些变化被NRG1A识别,导致其变构激活。我们进一步表明,抑制性NRG1家族成员NRG1C在与第二信使激活的EDS1-SAG101结合方面有效地优于NRG1A。这些发现揭示了NRG1A通过识别修饰的宿主EDS1-SAG101复合物而激活的机制,以及NRG1C通过隔离激活的EDS1-SAG101而抑制NRG1A的机制,从而揭示了中心植物免疫应答系统的激活和约束。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


