Gut IgA functionally interacts with systemic IgG to enhance antipneumococcal vaccine responses
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
Science Advances
Pub Date : 2025-02-12
引用次数: 0
Abstract
The gut microbiota enhances systemic immunoglobulin G (IgG) responses to vaccines, but it is unknown whether this effect involves IgA, which coats intestinal microbes. That IgA may amplify postimmune IgG production is suggested by the impaired IgG response to pneumococcal vaccines in some IgA-deficient patients. Here, we found that antipneumococcal but not total IgG production was impaired in mice with IgA deficiency. The positive effect of gut IgA on antipneumococcal IgG responses started very early in life and could implicate gut bacteria, as these responses were attenuated in germ-free mice recolonized with gut microbes from IgA-deficient donors. IgA could exert this effect by constraining the systemic translocation of gut antigens, which was associated with chronic immune activation, including T cell overexpression of programmed cell death protein 1 (PD-1). This inhibitory receptor may attenuate antipneumococcal IgG production by causing B cell hyporesponsiveness, which improved upon anti–PD-1 treatment. Thus, gut IgA functionally interacts with systemic IgG to enhance antipneumococcal vaccine responses.
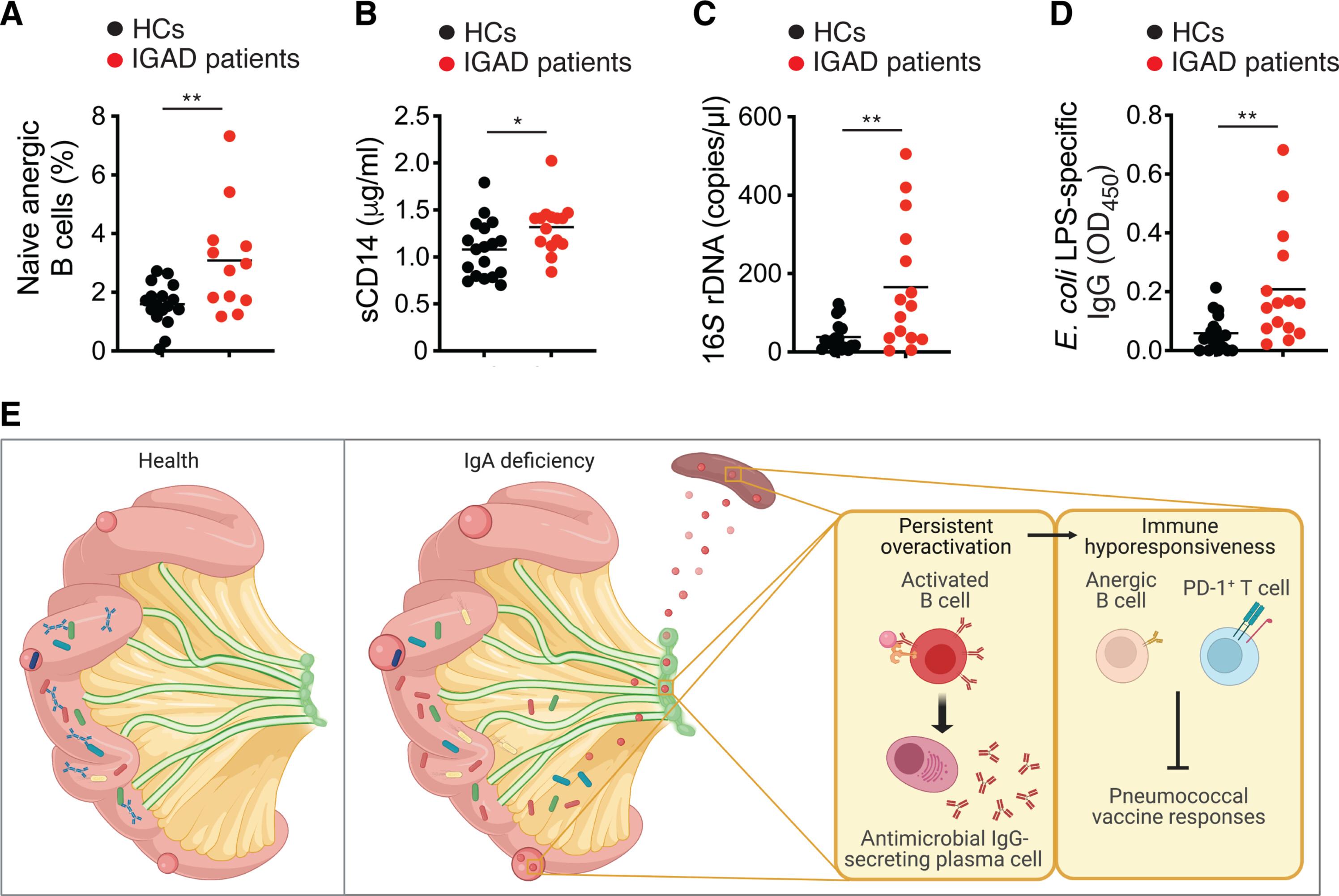
肠道IgA与全身IgG相互作用,增强抗肺炎球菌疫苗反应
肠道微生物群增强了全身免疫球蛋白G (IgG)对疫苗的反应,但尚不清楚这种作用是否涉及覆盖在肠道微生物上的IgA。在一些缺乏IgA的患者中,肺炎球菌疫苗对IgG的反应受损,表明IgA可能会增加免疫后IgG的产生。在这里,我们发现在IgA缺乏的小鼠中抗肺炎球菌而不是总IgG产生受损。肠道IgA对抗肺炎球菌IgG反应的积极作用在生命早期就开始了,可能与肠道细菌有关,因为这些反应在无菌小鼠中被来自缺乏IgA的供体的肠道微生物重新定殖。IgA可以通过抑制肠道抗原的系统性易位来发挥这种作用,这与慢性免疫激活有关,包括T细胞过表达程序性细胞死亡蛋白1 (PD-1)。这种抑制受体可能通过引起B细胞低反应性来减弱抗肺炎球菌IgG的产生,这种反应性在抗pd -1治疗后得到改善。因此,肠道IgA与全身IgG在功能上相互作用以增强抗肺炎球菌疫苗反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


