Activation Methods of Carboxyl Functions for Enhanced Aptamer Immobilization on Glassy Carbon for Application to Electrochemical Biosensing
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Electrochemical aptasensors are an attractive class of biosensors for target detection in several complex matrices. The immobilization procedure of the aptamers is currently one of the technological bottlenecks affecting biosensors’ performance. It must ensure both the preservation of its affinity toward the target and its stability. Herein, we evaluate carboxyl function activation methods for further aptamer immobilization in the design of glassy carbon-based aptasensors in a three steps strategy. Aptamer immobilization at the glassy carbon surface was conducted in three steps: (i) electrografting of diazonium salts for the functionalization of the electrode with carboxyl groups, (ii) activation of the carboxylic groups, and (iii) immobilization of a DNA aptamer sequence. We focused on the activation step of carboxylic groups by evaluating three coupling agents: the widely reported EDC/NHS carbodiimide agent, bromotripyrrolidinophosphonium hexafluorophosphate (PyBroP), and 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminium tetrafluoroborate (TBTU). Cyclic voltammetry, electrochemical impedance spectroscopy, X-ray photoelectron spectroscopy, and water contact angle were used to characterize and confirm each surface modification step, specifically the activation step, which, to our knowledge, has not been investigated before using these activation agents. Aminoferrocene was first used as an electroactive molecule to electrochemically evaluate its coupling with activated carboxylic groups using the different agents. The developed approach for designing this electrochemical aptasensor was subsequently applied to the immobilization of an aptamer sequence for the detection of diclofenac. This work is part of a proof-of-concept study that could be further developed for the design of an electrochemical aptasensor.
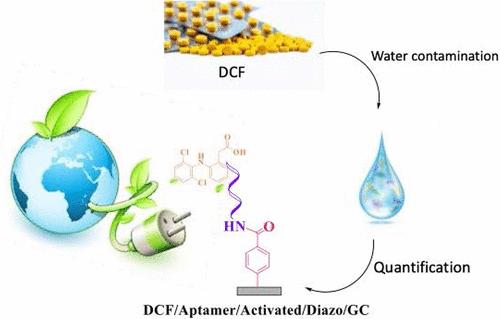
羧基活化法增强玻璃碳上适配体的固定化及其在电化学生物传感中的应用
电化学感应传感器是一类有吸引力的生物传感器,用于多种复杂基质的目标检测。适配体的固定过程是目前影响生物传感器性能的技术瓶颈之一。它必须保证对目标的亲和力和稳定性。在此,我们评估羧基功能激活方法,以进一步在玻璃碳基适配体传感器设计中的适配体固定化。适体在玻碳表面的固定分三个步骤进行:(i)重氮盐电接枝使电极与羧基功能化;(ii)羧基活化;(iii) DNA适体序列的固定。我们通过评估三种偶联剂来关注羧基的活化步骤:广泛报道的EDC/NHS碳二亚胺剂,溴三吡咯二磷六氟磷酸(PyBroP)和2-(1h -苯并三唑-1-基)-1,1,3,3-四氟硼酸四甲基胺(TBTU)。循环伏安法、电化学阻抗谱、x射线光电子能谱和水接触角被用来表征和确认每个表面修饰步骤,特别是活化步骤,据我们所知,在使用这些活化剂之前,还没有研究过活化步骤。首先将氨基二铁二烯作为电活性分子,用不同的试剂对其与活性羧基的偶联进行了电化学评价。该电化学适体传感器的设计方法随后应用于双氯芬酸检测适体序列的固定化。这项工作是概念验证研究的一部分,可以进一步开发电化学感应传感器的设计。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


