Investigating the Electric Double-Layer Structures between a Pt Electrode and Water/Acetonitrile Hybrid Electrolytes
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
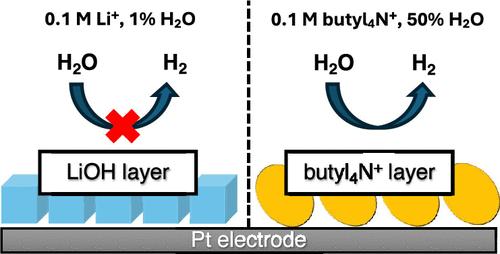
Controlling the reactivity of water at electrocatalytic interfaces is a critical challenge in many electrocatalytic reactions. Its reactivity can be adjusted by altering the composition of hybrid aqueous/organic electrolytes. To advance this approach, it is essential to understand how the structure of the electrode/hybrid electrolyte interface depends upon the electrode potential. This understanding is largely lacking. Herein, using surface-enhanced infrared absorption spectroscopy (SEIRAS), we probed the interfaces formed between a Pt electrode and acetonitrile/water mixtures containing 0.1 M LiClO4 or (butyl4N)ClO4. In the presence of Li+ and with decreasing potential, crystalline LiOH deposits on the electrode in electrolytes with a low water content (∼1% by weight) due to the low solubility of this salt in acetonitrile, blocking the active sites of the hydrogen evolution reaction (HER). In the presence of butyl4N+, the surface becomes more hydrophobic with decreasing potential. Notably, butyl4N+ ions form an irreversibly physisorbed adlayer solely in electrolytes with a high water content. Despite the formation of the adlayer, the electrode remains active for the HER.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


