Multienzyme-Activity Sulfur Quantum Dot Nanozyme-Mediated Cascade Reactions in Whole-Stage Symptomatic Therapy of Infected Bone Defects
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Integrating the therapeutic efficacy of early bacterial clearance, midstage inflammatory remission, and late-stage effective tissue healing is considered a pivotal challenge in symptomatic treatment of infected bone defects (IBDs). Herein, a microenvironment-adaptive nanoplatform based on a sulfur quantum dot (SQD) nanozyme was proposed for whole-stage symptomatic therapy of IBDs by mediating the sequence of enzyme cascade reactions. The SQD nanozyme prepared by a size-engineering modification strategy exhibits enhanced multienzyme activity compared to conventional micrometer- and nanometer-sized sulfur particles. In the early stages of bacterial infection, the SQD nanozyme self-activates superoxide dismutase-peroxidase activity, resulting in the production of reactive oxygen species (ROS) that effectively eliminate bacteria. After disinfection, the SQD nanozyme self-switched to superoxide dismutase-catalase mimetic behavior and eliminated excess ROS, efficiently promoting macrophage polarization to an anti-inflammatory phenotype in the midinflammatory microenvironment. Importantly, SQD nanozyme-mediated M2 macrophage polarization significantly improved the damaged bone immune microenvironment, accelerating bone repair at late-stage tissue healing. Therefore, this strategy offers a promising and viable approach for the treatment of infectious tissue healing by developing multienzyme-activity nanozymes that respond intelligently to the microenvironment at different stages, effectively fighting bacteria, reducing inflammation, and promoting tissue regeneration for whole-stage symptomatic therapy.
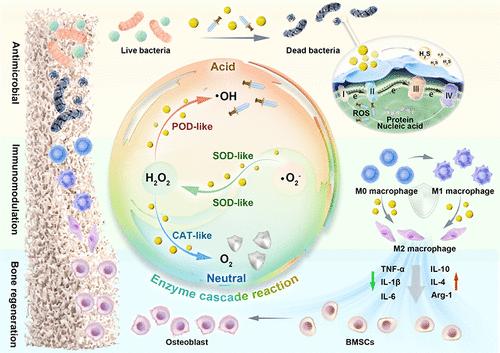
多酶活性硫量子点纳米酶介导的级联反应在感染性骨缺损全期对症治疗中的应用
整合早期细菌清除、中期炎症缓解和晚期有效组织愈合的治疗效果被认为是感染性骨缺损(IBDs)对症治疗的关键挑战。本文提出了一种基于硫量子点(SQD)纳米酶的微环境自适应纳米平台,通过介导酶级联反应序列,用于ibd的全阶段对症治疗。与传统的微米级和纳米级硫颗粒相比,通过尺寸工程修饰策略制备的SQD纳米酶具有更强的多酶活性。在细菌感染的早期阶段,SQD纳米酶自我激活超氧化物歧化酶-过氧化物酶活性,导致活性氧(ROS)的产生,有效地消灭细菌。消毒后,SQD纳米酶自我切换到超氧化物歧化酶-过氧化氢酶模拟行为,消除多余的ROS,有效促进巨噬细胞极化到炎症微环境中的抗炎表型。重要的是,SQD纳米酶介导的M2巨噬细胞极化显著改善了受损骨的免疫微环境,加速了后期组织愈合时的骨修复。因此,该策略为治疗感染性组织愈合提供了一种有希望和可行的方法,通过开发多酶活性纳米酶,在不同阶段对微环境做出智能反应,有效地对抗细菌,减少炎症,促进组织再生,进行全阶段对症治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


