Azole-Based Diarylethenes Containing Benzoheteroarene π-Linkers for Solar Thermal Energy Storage: Influence of Aromaticity and Noncovalent Interactions
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
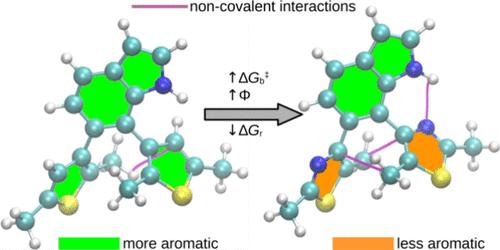
Diarylethene photoswitches featuring azole-based diaryl units combined with benzoheteroarene π-linkers have gained significant research interest in recent years due to their potential to achieve higher photocyclization efficiencies compared to conventional dithienylethene switches. In this work, we investigate the suitability of these photoswitches for molecular solar thermal energy storage (MOST) applications through computational modeling of their electrocyclization and cycloreversion reactions. Our calculations demonstrate that it is possible to achieve simultaneously both large energy-storage densities (0.29–0.35 MJ kg–1) and prolonged energy-storage times (half-lives of up to 124 days) under ambient conditions in dithiazolyl and dioxazolyl switches containing six distinct benzoheteroarene π-linkers. Furthermore, isomerization stabilization energy calculations and noncovalent interaction analysis reveal that the variations in energy-storage densities and times between the azole-based and dithienylethene switches stem from differences in aromaticities of the diaryl core and π-linker, as well as changes in noncovalent interactions. Notably, we demonstrate that the relative populations of photoreactive anti-parallel and non-photoreactive parallel conformers of the ring-open form of these switches are governed by weak intramolecular C···C interactions between the two aryl rings. These findings highlight the importance of optimizing such interactions to enhance energy-storage efficiencies in MOST systems.
含苯并杂芳烃的氮基二乙烯类太阳能储能π-连接剂:芳香性和非共价相互作用的影响
由于与传统的二乙烯开关相比,二乙烯开关具有更高的光循环效率,因此近年来,以氮基二芳基单元与苯并杂芳烃π连接剂结合的二乙烯光开关获得了很大的研究兴趣。在这项工作中,我们通过对其电环化和环还原反应的计算建模来研究这些光开关在分子太阳能热储能(MOST)应用中的适用性。我们的计算表明,在环境条件下,含有六种不同的苯并杂二烯π连接剂的二噻唑和二恶唑开关可以同时实现大的储能密度(0.29-0.35 MJ kg-1)和延长的储能时间(半衰期高达124天)。此外,异构化稳定能计算和非共价相互作用分析表明,氮基和二乙烯基开关的蓄能密度和蓄能时间的差异源于二芳基核和π连接体的芳香性差异以及非共价相互作用的变化。值得注意的是,我们证明了这些开关的开环形式的光反应反平行和非光反应平行构象的相对居群是由两个芳基环之间的弱分子内C···C相互作用控制的。这些发现强调了优化这种相互作用以提高MOST系统储能效率的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


