Electrocyclization of Arylvinyl Oxetane/1,3-Diol to Substituted Indenyl Acetaldehyde via Indene–Ethanol, Oxepine, and a 1,6-Hydride Shift
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
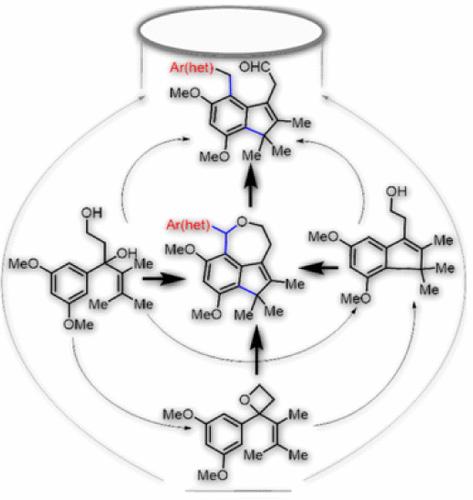
We intended to realize aryl vinyl oxetane as a 4π-electrocyclization precursor to access indene ethanol. Interestingly, we found that the readily accessible aryl vinyl 1,3-diol, an intermediate en route to the synthesis of oxetane, is an equally potential precursor for the anticipated cyclization. Moreover, aryl vinyl 1,3-diol/oxetane and indene ethanol readily reacted with the subsequently added (het)aromatic aldehydes, providing various indene oxepines/acetaldehydes. Additionally, indenyl aldehyde served as a synthetic handle for FGI, further expanding this protocol’s scope.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


