Heavier alkaline earth and heterobimetallic s-block “ate” complexes of a di(amido)siloxane ligand: solid-state structure and dynamic solution-phase behaviour
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
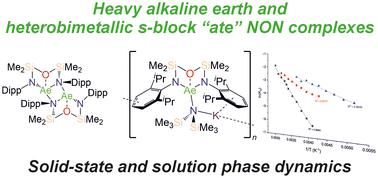
The diverse solid-state structures and solution-phase dynamics of both neutral and heterometallic s-block “ate” complexes of the heavier alkaline earth metals (Ae; Ca–Ba) supported by a chelating and flexible di(amido)siloxane ligand ([NON-DippL]2− = [O(SiMe2NDipp)2]2−) are described, enabling comparison with those of closely related di(amido) ligands based on either flexible aliphatic or rigid xanthene-based backbones. Three dimeric alkaline earth complexes [(NON-DippL)Ae]2 (Ae = Ca (2), Sr (3) and Ba (4)) which feature a κ3-N,O,N′-κ1-N′-tridentate coordination mode were prepared from protonolysis reactions between NON-DippLH2 with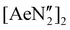 (Ae = Ca, Sr and Ba); N′′ = [N(SiMe3)2]−. In tetrahydrofuran, these complexes were readily converted into the monomeric adducts [(NON-DippL)Ae(thf)n] (n = 2, Ae = Ca (5); n = 3, Ae = Sr (6) and Ba (7)). Heterometallic Ae/K amide “ate” complexes were afforded through two routes: reaction of previously reported [(NON-DippL)Mg]2 (1) with two equivalents of KN′′ at elevated temperatures resulted in [(NNO-DippL)Mg(μ-N′′)K]n (8; NNO-DippL = [OSiMe2NDippSiMe2NDipp]2−), whereas the equimolar reaction of NON-DippLH2 with
(Ae = Ca, Sr and Ba); N′′ = [N(SiMe3)2]−. In tetrahydrofuran, these complexes were readily converted into the monomeric adducts [(NON-DippL)Ae(thf)n] (n = 2, Ae = Ca (5); n = 3, Ae = Sr (6) and Ba (7)). Heterometallic Ae/K amide “ate” complexes were afforded through two routes: reaction of previously reported [(NON-DippL)Mg]2 (1) with two equivalents of KN′′ at elevated temperatures resulted in [(NNO-DippL)Mg(μ-N′′)K]n (8; NNO-DippL = [OSiMe2NDippSiMe2NDipp]2−), whereas the equimolar reaction of NON-DippLH2 with 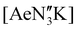 led to [(NON-DippL)Ae(μ-N′′)K]n (Ae = Ca (9), Sr (10) and Ba (11)). Complexes 8–11 exist as one-dimensional coordination polymers propagated by K+–aryl π-facial interactions in the solid-state. The mixed amide/siloxide “NNO” ligand in 8 results from a 1,3-silyl retro-Brook rearrangement of the original di(amido)siloxane ligand, while the larger Ae2+ congeners readily accommodate the coordination of KN′′ with the di(amido)siloxane ligand retaining a κ3-N,O,N′-tridentate motif in 9–11. Finally, the solution-phase behaviour of 8–11 in both toluene and thf were investigated indicating the reversible dissociation of KN′′ from 9–11 and the thermodynamic parameters of this process were elucidated.
led to [(NON-DippL)Ae(μ-N′′)K]n (Ae = Ca (9), Sr (10) and Ba (11)). Complexes 8–11 exist as one-dimensional coordination polymers propagated by K+–aryl π-facial interactions in the solid-state. The mixed amide/siloxide “NNO” ligand in 8 results from a 1,3-silyl retro-Brook rearrangement of the original di(amido)siloxane ligand, while the larger Ae2+ congeners readily accommodate the coordination of KN′′ with the di(amido)siloxane ligand retaining a κ3-N,O,N′-tridentate motif in 9–11. Finally, the solution-phase behaviour of 8–11 in both toluene and thf were investigated indicating the reversible dissociation of KN′′ from 9–11 and the thermodynamic parameters of this process were elucidated.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dalton Transactions
化学-无机化学与核化学
CiteScore
6.60
自引率
7.50%
发文量
1832
审稿时长
1.5 months
期刊介绍:
Dalton Transactions is a journal for all areas of inorganic chemistry, which encompasses the organometallic, bioinorganic and materials chemistry of the elements, with applications including synthesis, catalysis, energy conversion/storage, electrical devices and medicine. Dalton Transactions welcomes high-quality, original submissions in all of these areas and more, where the advancement of knowledge in inorganic chemistry is significant.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


