Impact of Metal Species on Chain Transfer Mode in Bis(imino)pyridyl Complexes Catalyzed Ethylene Polymerization
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
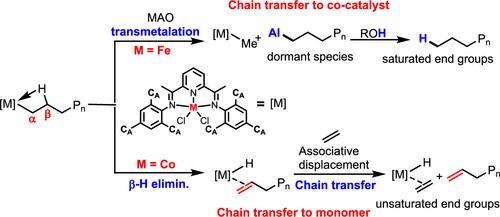
Chain transfer reactions are pivotal in coordination polymerization, influencing catalytic efficiency, molecular weight adjustment, and control over the chain-end structure. In this study, we present the synthesis and characterization of two bis(imino)pyridyl ligands featuring flexible o-aryl cycloalkyl substituents, along with their corresponding iron(II) and cobalt(II) complexes. These complexes exhibited very high polymerization activities, reaching up to 1.8 × 107 g mol–1 h–1, and produced highly linear polyethylene with a wide range of molecular weights (MW = 2.1–232.8 kg/mol) during ethylene polymerization. The sizes of the cycloalkyl ring and the metal species were found to have a substantial impact on the polymerization process. Specifically, smaller cyclopentyl substituents enhanced polymerization activity and increased the molecular weight of the resulting polyethylene for both iron(II) and cobalt(II) complexes compared to their cyclohexyl counterparts. When complexes with the same ligand were compared, iron(II) species generated polyethylene with higher molecular weights and broader molecular weight distributions in comparison to the corresponding cobalt(II) species under identical conditions. The most striking finding is the significant selectivity of the metal species in our system for different chain transfer modes. Analysis of the molecular weight distributions and chain-end structures of the resulting polyethylene revealed that chain transfer to the cocatalyst MAO predominates in iron(II)-catalyzed ethylene polymerization, whereas in cobalt(II)-catalyzed ethylene polymerization, chain transfer to the monomer ethylene is the primary mode.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


