Blocking S1P4 signaling attenuates brain injury in mice with ischemic stroke
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
The functions of S1P receptors have been revealed using genetic and pharmacological tools, including the potent non-selective modulator FTY720. However, studies on subtype-specific agonists and antagonists are limited; hence, the role of S1P4 remains unclear.Objectives
To identify a novel function of S1P4 as a pathogenic factor in stroke using a newly developed S1P4-selective modulator and S1P4 knockdown.Methods
Heteroaromatic analogs of FTY720 were synthesized, a β-arrestin assay was conducted against S1P receptors, and the developed compound (NXC736) was characterized as a functional S1P4 antagonist. To clarify the function of S1P4, the therapeutic potential of NXC736 in ischemic stroke was determined using a transient middle cerebral artery occlusion (tMCAO) mouse model, which was validated using S1P4 knockdown. The S1P4-dependent pathogenic mechanisms were determined using immunohistochemical and biochemical analyses.Results
Molecular modeling studies provide valuable clues for understanding S1P4 selectivity of NXC736. NXC736 contains a triazole ring instead of a phenyl ring and exhibits S1P4-selective activity as a functional antagonist. Its action on S1P4 does not require phosphorylation by sphingosine kinase 2. Notably, NXC736 exhibited substantial therapeutic activity against ischemic stroke by attenuating tMCAO-induced acute brain injuries, including brain infarction, neurological deficits, and neuronal apoptosis. This suggested that S1P4 is a pathogenic factor in ischemic stroke. This function was confirmed using AAV-based S1P4 knockdown. NXC736 or S1P4 knockdown attenuated blood–brain barrier disruption, neutrophil infiltration, microglial activation and proliferation, and the upregulation of pro-inflammatory cytokines, thereby demonstrating that S1P4 influences neuroinflammatory responses in ischemic stroke. The underlying mechanisms were activation of NLRP3 inflammasome, NF-κB, and MAPKs. S1P4 also contributed to chronic brain injuries caused by ischemic stroke because NXC736 exerted long-term neuroprotective effects against tMCAO challenge.Conclusion
Using a functional S1P4 antagonist (NXC736) and a genetic tool for S1P4 knockdown, we identified S1P4 as a novel pathogenic factor in ischemic stroke.
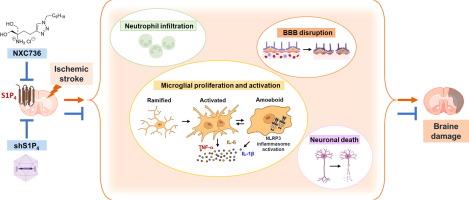
阻断S1P4信号可减轻缺血性脑卒中小鼠脑损伤
引言利用基因和药理学工具(包括强效非选择性调节剂 FTY720)揭示了 S1P 受体的功能。方法合成了 FTY720 的杂芳香族类似物,进行了针对 S1P 受体的 β-restin 检测,并将所开发的化合物(NXC736)表征为功能性 S1P4 拮抗剂。为了明确 S1P4 的功能,研究人员利用瞬时大脑中动脉闭塞(tMCAO)小鼠模型确定了 NXC736 对缺血性中风的治疗潜力,并通过 S1P4 基因敲除进行了验证。结果分子建模研究为了解 NXC736 的 S1P4 选择性提供了有价值的线索。NXC736 含有一个三唑环而不是一个苯基环,作为一种功能性拮抗剂具有 S1P4 选择性活性。它对 S1P4 的作用不需要鞘磷脂激酶 2 的磷酸化。值得注意的是,NXC736 通过减轻 tMCAO 引起的急性脑损伤(包括脑梗塞、神经功能缺损和神经细胞凋亡),对缺血性中风具有显著的治疗活性。这表明 S1P4 是缺血性中风的致病因子。这一功能通过基于 AAV 的 S1P4 基因敲除得到了证实。NXC736 或 S1P4 基因敲除减轻了血脑屏障破坏、中性粒细胞浸润、小胶质细胞活化和增殖以及促炎细胞因子的上调,从而证明 S1P4 影响缺血性中风的神经炎症反应。其潜在机制是激活 NLRP3 炎性体、NF-κB 和 MAPKs。结论利用功能性 S1P4 拮抗剂(NXC736)和基因工具敲除 S1P4,我们发现 S1P4 是缺血性中风的新型致病因素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


