Elucidation of the Activity and pH Stability Limits of Polyoxometalate-Intercalated Layered Double Hydroxide Nanocomposites toward Water Oxidation Catalysis
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
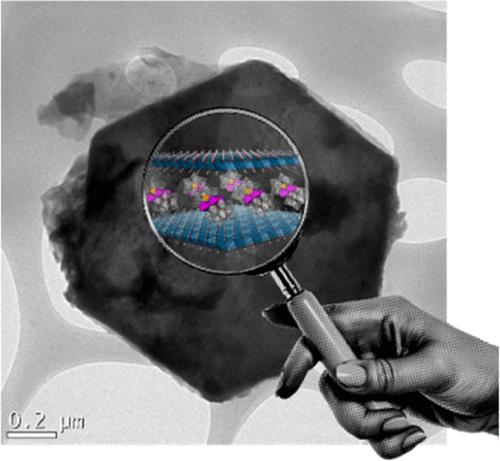
The inclusion of water oxidation active polyoxometalates (POMs) inside layered materials is a promising strategy to increase their catalytic efficiency while overcoming their fragility under homogeneous conditions. In this sense, intercalation of POMs in the interlaminar space of layered double hydroxides (LDHs), formed by positively charged brucite-type inorganic layers, is a very interesting strategy that is gaining attention in the field. Despite their huge potential, there is a lack of accurate characterization of the materials, especially after their use as water oxidation catalysts under pH conditions in which the POM counterpart has been demonstrated to be unstable (strong alkali media). For this reason and as a proof of concept, we have intercalated the well-known [Co4(H2O)2(PW9O34)2]10– POM (Co4-POM) in the lamellar space of the Mg2Al-LDH, to study its catalytic capabilities and stability. Remarkably, the nanocomposites show improved water oxidation efficiencies with excellent stability in close-to-neutral media compared with the water-insoluble cesium salt of Co4-POM or commercial Co3O4. However, thorough postcatalytic characterization of the nanocomposites demonstrates that the polyoxotungstate framework of the POM suffers from hydrolytic instability in strong alkali conditions, leading to the formation of a mixed-valence cobalt(II/III) oxide in the interlayer space of Mg2Al-LDH. This work highlights the importance of accurately assessing the fate of the catalytic POM after the catalytic reaction, especially when conditions are employed outside the pH stability window of the POM, which can lead to erroneous conclusions and mistaken catalytic activities.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


