Sustainable Synthesis of MCM-22 Zeolite as a Catalytic Platform for Propane Dehydrogenation
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The significant volume of solvent required for the hydrothermal synthesis of zeolites remains the primary hurdle impeding industrial applications. With the benefits of reduced manufacturing costs, safety, and energy savings, reducing the use of solvents is one of the significant sought-after objectives. In this study, borosilicate zeolite B-MCM-22 is successfully obtained using a solvent-free synthesis method. The as-synthesized sample exhibits good long-range order, high crystallinity, and high silica utilization (ca. 95%). Furthermore, the B-MCM-22 precursor serves as a platform for introducing Co active sites via deboronization and impregnation processes. The resulting Co-MCM-22-DB catalyst provides a propylene selectivity of >90% with an initial propane conversion of 38% in the propane dehydrogenation reaction and shows a higher initial catalytic performance than the Co/ITQ-1 catalyst. The essential interplay between the metal–support interaction and catalytic performance is demonstrated by ultraviolet–visible (UV–vis), H2-TPR, and catalytic assessments, further elucidating that Co2+ species are the active sites for propane to propylene conversion. We anticipate that B-MCM-22 will be a versatile and ideal platform for catalyst design through structural manipulation.
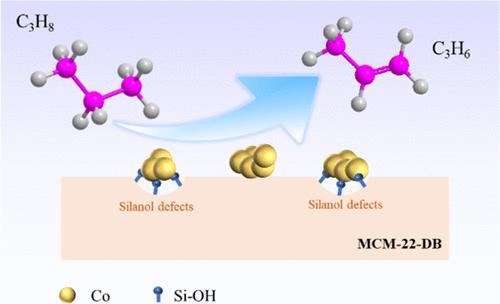
可持续合成 MCM-22 沸石作为丙烷脱氢的催化平台
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


