Structural Stability and Photoluminescence Property of Cs2UCl6 Single Crystal Derived from Spent Nuclear Fuel
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
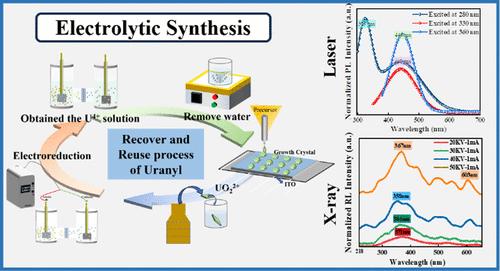
The recycling and reuse of trace uranium from spent nuclear fuel is of great significance for the safety management of the nuclear fuel cycle. However, stabilization of low-valent uranium has always been a challenge due to the ultraoxidizable nature of uranium ions, which remains relatively uncharted territory in spent fuel treatment. In the current study, U4+ was immobilized in Cs2UCl6 single crystal with a perovskite structure from uranyl under a strong acidic environment. A comprehensive and detailed understanding of Cs2UCl6 at the atomic scale has been achieved by combining density functional theory (DFT) with high-resolution integrated differential phase contrast scanning transmission electron microscopy (iDPC-STEM) imaging, which was captured by utilizing Cs-corrected TEM for the first time. Furthermore, the results obtained from X-ray excitation and the photoexcitation effects produced by PL at 280, 330, and 360 nm provide compelling evidence for the ability of U4+ to form excitable bands around the Fermi level. The as-synthesized Cs2UCl6 demonstrates excellent thermal stability above 275 °C, as evidenced by in situ Raman spectroscopy and thermogravimetric analysis, while a degradation pathway initiated by CsCl upon exposure to water vapor was revealed by synchrotron X-ray diffraction. Thermal and chemical stability can be further elevated by consolidating it into a metal–organic framework (MOF) via hot pressing. The current study provides a promising strategy to reuse and functionalize the spent nuclear fuel.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


