Highly reactive ZnFe2O4/TiO2 p-n heterojunction photocatalyst accelerates interfacial charge transfer for boosted photodegradation of ammonia nitrogen
IF 4.1
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
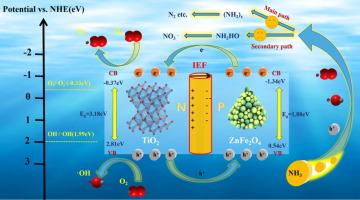
Excessive ammonia nitrogen (NH4+-N) in wastewater worsens the living conditions of aquatic organisms and causes chronic poisoning of humans. In this work, the ZnFe2O4/TiO2 p-n heterojunction photocatalyst was prepared to remove NH4+-N. ZT-10 (the molar ratio of ZnFe2O4 to TiO2 was 1:10) removed 98.52 % of NH4+-N (50 mg/L) at pH 10 in 90 min with a stabilized crystal structure maintained over four cycles. The experiment results demonstrated universal adaptability to various representative contaminants in actual waters. The meaningful roles of superoxide radical (∙O2−), hydroxyl radical (∙OH), and holes (h+) in NH4+-N photoreduction were confirmed by radical trapping experiments. The photocatalytic mechanism revealed that the improvement of photodegradation activity was primarily due to the internal electric field (IEF) generated at the p-n heterojunction interface, which facilitated the precise migration and aggregation of photoinduced electrons (e-) and holes to space reaction sites, realizing the highly efficient surface catalysis of ZnFe2O4/TiO2 photocatalysts.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


