ArfGAP2 promotes STING proton channel activity, cytokine transit, and autoinflammation
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
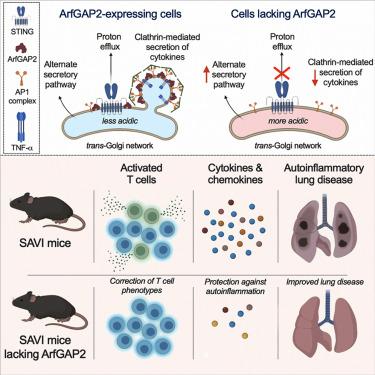
Stimulator of interferon genes (STING) transmits signals downstream of the cytosolic DNA sensor cyclic guanosine monophosphate-AMP synthase (cGAS), leading to transcriptional upregulation of cytokines. However, components of the STING signaling pathway, such as IRF3 and IFNAR1, are not essential for autoinflammatory disease in STING gain-of-function (STING-associated vasculopathy with onset in infancy [SAVI]) mice. Recent discoveries revealed that STING also functions as a proton channel that deacidifies the Golgi apparatus. Because pH impacts Golgi enzyme activity, protein maturation, and trafficking, we hypothesized that STING proton channel activity influences multiple Golgi functions. Here, we show that STING-mediated proton efflux non-transcriptionally regulates Golgi trafficking of protein cargos. This process requires the Golgi-associated protein ArfGAP2, a cell-type-specific dual regulator of STING-mediated proton efflux and signaling. Deletion of ArfGAP2 in hematopoietic and endothelial cells markedly reduces STING-mediated cytokine and chemokine secretion, immune cell activation, and autoinflammatory pathology in SAVI mice. Thus, ArfGAP2 facilitates STING-mediated signaling and cytokine release in hematopoietic cells, significantly contributing to autoinflammatory disease pathogenesis.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


