Resistance to Striga parasitism through reduction of strigolactone exudation
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
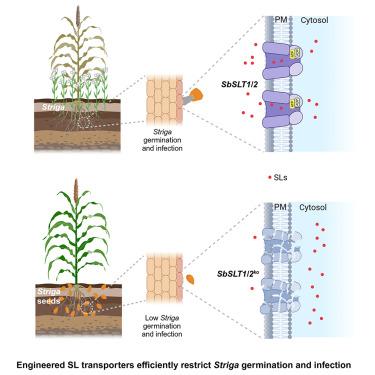
Parasitism with Striga poses a major threat to global food production. Striga germination and growth rely on strigolactones (SLs) exuded by crop roots under phosphate (Pi)-deficient conditions, although the mechanism of this host-parasite interaction remains elusive. In this study, transcriptomic and functional analyses of sorghum treated with Pi deficiency or the SL GR245DS identify two ABC transporter G (ABCG) transporters of SL, Sorghum biocolor strigolactones transporter 1 (SbSLT1) and SbSLT2. Using AlphaFold2 and amino acid conversion mutants, we identify highly conserved amino acids in SL transport channels essential for transport function. Sorghum lines with single or double knockouts of these transporters exhibit significantly reduced SL secretion from roots, leading to decreased Striga germination and parasitism in field experiments and consequently reducing the grain loss under Striga infestation. This study thus describes the mechanism of SL exudation in monocots and defines conserved residues essential for SL transporter function, offering a potential strategy for enhancing crop resistance to Striga parasitism.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


