Symbionts of predatory protists are widespread in the oceans and related to animal pathogens
IF 20.6
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Protists are major predators of ocean microbial life, with an ancient history of entanglements with prokaryotes, but their delicate cell structures and recalcitrance to culturing hinder exploration of marine symbioses. We report that tiny oceanic protistan predators, specifically choanoflagellates—the closest living unicellular relatives of animals—and uncultivated MAST-3 form symbioses with four bacterial lineages related to animal symbionts. By targeting living phagotrophs on ship expeditions, we recovered genomes from physically associated uncultivated Legionellales and Rickettsiales. The evolutionary trajectories of Marinicoxiellaceae, Cosmosymbacterales, Simplirickettsiaceae, and previously named Gamibacteraceae vary, including host-engagement mechanisms unknown in marine bacteria, horizontally transferred genes that mediate pathogen-microbiome interactions, and nutritional pathways. These symbionts and hosts occur throughout subtropical and tropical oceans. Related bacteria were detected in public data from freshwater, fish, and human samples. Symbiont associations with animal-related protists, alongside relationships to animal pathogens, suggest an unexpectedly long history of shifting associations and possibilities for host expansion as environments change.
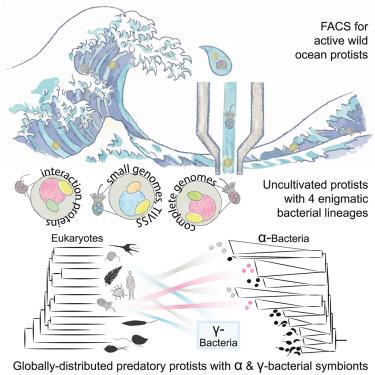
掠食性原生生物的共生体在海洋中广泛存在,与动物病原体有关
原生生物是海洋微生物生命的主要捕食者,与原核生物纠缠的历史悠久,但其微妙的细胞结构和对培养的抗拒阻碍了海洋共生的探索。我们报道了微小的海洋原生捕食者,特别是鞭毛虫——动物最亲近的单细胞亲戚——和未培养的mat -3与四种与动物共生体相关的细菌谱系形成共生。通过在船舶探险中瞄准活的吞噬菌,我们从物理上相关的未培养军团菌和立克次体中恢复了基因组。Marinicoxiellaceae, Cosmosymbacterales, simpliricketttsiaceae和先前命名的Gamibacteraceae的进化轨迹各不相同,包括海洋细菌中未知的宿主参与机制,介导病原体-微生物相互作用的水平转移基因以及营养途径。这些共生体和寄主遍布亚热带和热带海洋。在淡水、鱼类和人体样本的公开数据中检测到相关细菌。与动物相关的原生生物的共生关系,以及与动物病原体的关系,表明随着环境的变化,宿主之间的关系和扩展的可能性出乎意料地长。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


