Palladium-catalyzed domino cyclization and C–H amination of 1,7-enynes toward N-containing fused quinolin-2(1H)-ones†
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A palladium-catalyzed domino radical cyclization and C–H amination of 1,7-enynes has been developed for the rapid establishment of N-containing fused quinolin-2(1H)-one scaffolds. The reaction of 1,7-enynes with perfluoroalkyl iodides and di-t-butyldiaziridinone worked well to give a wide range of N-containing fused quinolin-2(1H)-one derivatives in high yields. Notably, this method could be used in the late-stage modifications of various drugs.
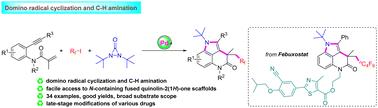
钯催化1,7-炔的多米诺环化和C-H胺化制备含n的融合喹啉-2(1H)-酮。
采用钯催化1,7-炔的多米诺骨牌自由基环化和C-H胺化,快速建立了含n-融合喹啉-2(1H)- 1支架。1,7-炔与全氟烷基碘化物和二叔丁基二氮吡啶酮反应良好,得到了多种含n的融合喹啉-2(1H)- 1衍生物,收率高。值得注意的是,该方法可用于各种药物的后期修饰。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


