MmoD and MmoG Are Crucial for the Synthesis of Soluble Methane Monooxygenase in Methanotrophs
IF 2.6
2区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Soluble methane monooxygenase (sMMO) from methanotrophs has been extensively investigated for decades. However, major knowledge gaps persist regarding the synthesis mechanism of sMMO, particularly concerning the ambiguous roles of mmoD and mmoG in the sMMO gene cluster. Here, the functions of mmoD and mmoG were investigated in a model methanotrophic strain, Methylotuvimicrobium buryatense 5GB1C. Both genes were found to be essential for the functional expression of sMMO. Genetic and biochemical data supported the hypothesis that MmoG acts as a folding chaperone for both MmoX and MmoR, while MmoD serves as an assembly chaperone for the hydroxylase component. The functional expression of sMMO in Escherichia coli was achieved in an mmoD- and mmoG-dependent manner. In addition, deletion of mmoD dramatically reduced the transcription of the sMMO cluster in M. buryatense 5GB1C, implying that MmoD may regulate the sMMO cluster via an unknown mechanism. Knockout of neither mmoD nor mmoG abolished the essential feature of “copper switch”, indicating that they do not serve as the initial regulators of “copper switch”. These results demonstrate the crucial roles of mmoD and mmoG in sMMO synthesis and offer new insights into heterologous expression of sMMO.
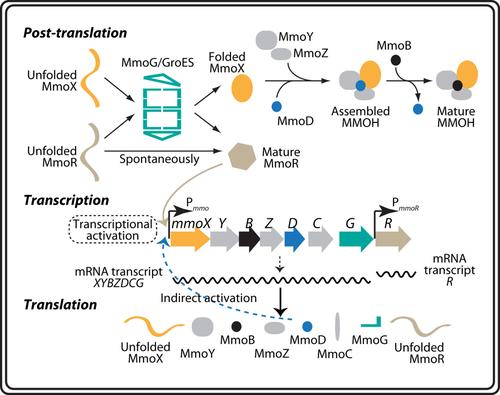
MmoD和MmoG是甲烷氧化菌合成可溶性甲烷单加氧酶的关键
甲醇氧化菌的可溶性甲烷单加氧酶(sMMO)已经被广泛研究了几十年。然而,关于sMMO的合成机制,特别是关于mmoD和mmoG在sMMO基因簇中的模糊作用,主要的知识差距仍然存在。本文研究了mmoD和mmoG在模型甲烷营养菌株buryatense Methylotuvimicrobium 5GB1C中的功能。这两个基因都是sMMO功能表达所必需的。遗传和生化数据支持MmoG作为MmoX和MmoR的折叠伴侣的假设,而MmoD作为羟化酶组分的组装伴侣。sMMO在大肠杆菌中以mmoD-和mmog依赖的方式实现功能表达。此外,mmoD的缺失显著降低了M. buryatense 5GB1C中sMMO簇的转录,这意味着mmoD可能通过未知的机制调节sMMO簇。敲除mmoD和mmoG都没有废除“铜开关”的本质特征,说明它们都不是“铜开关”的初始调节器。这些结果证明了mmoD和mmoG在sMMO合成中的重要作用,并为sMMO的异源表达提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Microbiology
生物-生化与分子生物学
CiteScore
7.20
自引率
5.60%
发文量
132
审稿时长
1.7 months
期刊介绍:
Molecular Microbiology, the leading primary journal in the microbial sciences, publishes molecular studies of Bacteria, Archaea, eukaryotic microorganisms, and their viruses.
Research papers should lead to a deeper understanding of the molecular principles underlying basic physiological processes or mechanisms. Appropriate topics include gene expression and regulation, pathogenicity and virulence, physiology and metabolism, synthesis of macromolecules (proteins, nucleic acids, lipids, polysaccharides, etc), cell biology and subcellular organization, membrane biogenesis and function, traffic and transport, cell-cell communication and signalling pathways, evolution and gene transfer. Articles focused on host responses (cellular or immunological) to pathogens or on microbial ecology should be directed to our sister journals Cellular Microbiology and Environmental Microbiology, respectively.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


