Nanovesicles for Lipid Metabolism Reprogram-Enhanced Ferroptosis and Magnetotherapy of Refractory Tumors and Inhibiting Metastasis with Activated Innate Immunity
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
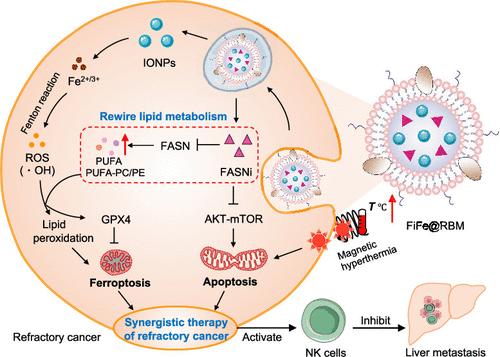
Castration-resistant prostate cancer (CRPC) is an intractable disease, but approaches for eradicating primary tumors and inhibiting metastasis are limited. Considering that lipid metabolism plays key roles in ferroptosis and tumor progression and treatment resistance, here we developed a biomimetic nanovesicle (FiFe@RBM) encapsulating fatty acid synthetase inhibitors and iron oxide nanoparticles for synergistic therapy of CRPC and inhibiting the metastasis. FiFe@RBM with superior magnetic properties efficiently delivered drugs into the CRPC cancer cells, where it can release Fe ions to efficiently induce reactive oxygen species and mitochondrial dysfunction and inhibit the AKT-mTOR pathway, which synergistically causes apoptosis and enhances ferroptosis by rewired lipid metabolism through increasing polyunsaturated fatty acids (PUFAs), PUFA-enriched phosphatidylcholine (PUFA-PC), PUFA-enriched phosphatidylethanolamine (PUFA-PE), etc. By intravenous injection, the high accumulation of FiFe@RBM in PC-3 tumors enabled precision T1/T2-weighted magnetic resonance imaging-guided effective eradication of human CRPC PC-3 tumors by synergistic magnetic hyperthermia therapy (MHT) and ferroptosis, which further inhibited liver metastasis by the activated and recruited high rates of natural killer cells in the nude mice model. This work presents an effective nanovesicle strategy for reprogramming lipid metabolism to enhance ferroptosis in synergy with MHT for effectively treating refractory cancers.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


