Revealing the regulation mechanism of carbon dots on Ni(OH)2 for optimizing methanol electrooxidation activity
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Rational design of Ni-based electrocatalysts for methanol electrooxidation reaction (MOR) and in-depth understanding of their catalytic mechanism remain a challenge. Herein, carbon dots/graphene heterostructure is constructed to load Ni(OH)2 and the role of carbon dots focuses on regulating distribution and electronic property of Ni(OH)2. Regulation mechanism of carbon dots on Ni(OH)2 for optimizing MOR activity are revealed by in-situ Raman, in-situ FTIR, operando electrochemical impedance spectroscopy, X-ray absorption fine structure (XAFS) spectroscopy, and so on. It’s found carbon dots could disperse Ni species and regulate their distribution configuration. Appropriate amount of carbon dots promotes the formation of highly dispersed CDs&Ni(OH)2 type. Because of strong interaction between Ni(OH)2 and CDs, coordination environment and local electronic structure of Ni in Ni-CDs/G can be regulated by carbon dots, leading to phase-ratio variation of α-Ni(OH)2/β‑Ni(OH)2 and d-band center shift of Ni(OH)2. For the best Ni-4CDs/G electrocatalyst, more formed α-Ni(OH)2 lead to d-band center downshift from the EF level and optimized its adsorption of methanol. Ni-4CDs/G shows the specific activity of 299.2 mA cm−2 and mass activity of 3756.5 mA mg−1 at peak current. In water splitting test, lower initial overpotential of 140 mV is found in methanol electrolysis than that in water electrolysis. This opens a new door to regulate the metal-based electrocatalysts by CDs and will inspire more people to explore CDs-based materials for electrocatalytic application.
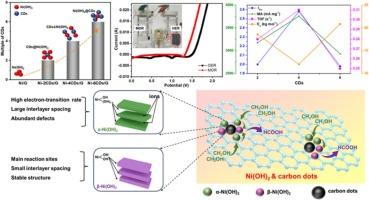

揭示碳点对Ni(OH)2优化甲醇电氧化活性的调控机理
合理设计用于甲醇电氧化反应(MOR)的镍基电催化剂和深入了解其催化机理仍然是一个挑战。本文构建了碳点/石墨烯异质结构来负载Ni(OH)2,碳点的作用主要是调节Ni(OH)2的分布和电子性质。利用原位拉曼光谱、原位红外光谱、电化学阻抗谱、x射线吸收精细结构(XAFS)光谱等研究方法揭示了碳点对Ni(OH)2的调控机制,以优化MOR活性。发现碳点可以分散镍元素并调节其分布构型。适量的碳点可促进高分散的CDs&;Ni(OH)2型的形成。由于Ni(OH)2与CDs之间的强相互作用,Ni在Ni-CDs/G中的配位环境和局部电子结构可以通过碳点调控,导致α-Ni(OH)2/β -Ni(OH)2的相比变化和Ni(OH)2的d波段中心偏移。对于最佳的Ni-4CDs/G电催化剂来说,α-Ni(OH)2的形成越多,导致其d波段中心从EF水平下移,并优化了其对甲醇的吸附。Ni-4CDs/G在峰值电流下的比活度为299.2 mA cm−2,质量活度为3756.5 mA mg−1。在解水试验中,甲醇电解的初始过电位为140 mV,低于水电解。这为利用cd调控金属基电催化剂打开了一扇新的大门,也将激励更多人探索基于cd的电催化材料的应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


