Redox-responsive micellar nanoparticles using benzothiazole-disulfide terminated polymers: employing host–guest complexation for targeted delivery of curcumin†
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
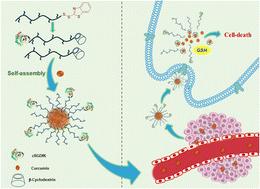
A host–guest interaction-based redox-responsive polymeric nanoparticle system for targeted delivery of curcumin is fabricated. Host–guest interaction between a β-cyclodextrin (β-CD) terminated hydrophilic polymer and curcumin is employed to formulate stable nanosized aggregates. A redox-sensitive disulfide linkage is used to tether the CD moiety onto the polymer chain ends to obtain efficient release inside cancerous cells. For the synthesis of the β-CD terminated redox-responsive polymer, a polyethylene glycol (PEG)-based polymer containing a benzothiazole-disulfide (BDS) group at the chain end was utilized. The BDS-terminated polymers were synthesized using reversible addition–fragmentation chain transfer (RAFT) polymerization by using a BDS-containing chain-transfer agent (CTA). Initially, the post-polymerization modification abilities of these polymers were demonstrated by successful conjugation of model thiol functional compounds such as furfuryl thiol and Bodipy-SH, a fluorescent hydrophobic dye. To prepare a nanoaggregate forming polymer precursor, a thiol functional β-CD host moiety was conjugated to the BDS end groups of the reactive polymer through redox-responsive disulfide linkage formation. The other end of the β-CD-containing polymer was capped with an integrin-targeting cyclic peptide (cRGDfK). While no aggregates were observed in the aqueous medium for the BDS-containing parent polymer, the β-CD attached polymers gave nanosized aggregates with average sizes below 150 nm. Curcumin-loaded nanoaggregates were obtained through the inclusion complexation of curcumin molecules with β-CD moieties. It was shown that enhanced drug release occurred upon exposure of the nanoaggregates to the glutathione (GSH) environment. While the parent BDS functional polymers and β-CD-containing polymeric aggregates were non-toxic to healthy fibroblast cells, curcumin-loaded aggregates showed dose-dependent toxicity against the U-87 glioblastoma cancer cell line. The peptide-targeting group containing nanoparticles showed slightly higher toxicity and enhanced cellular internalization. The modular nanoparticle system disclosed here could be employed to address challenges in the delivery of hydrophobic drugs to cancer cells.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


