Autophagy-Activating Nanoautophagosome-Tethering Compounds for Targeted Protein Degradation Specifically in Tumor Cells
IF 5.1
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
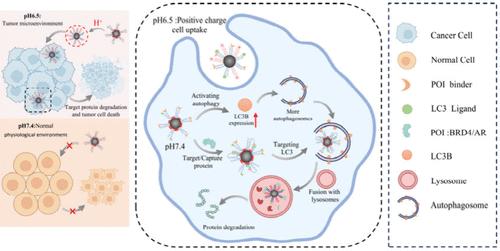
Autophagosome-tethering compounds (ATTECs) represent an emerging targeted protein degradation (TPD) technology that directly draws intracellular proteins of interest (POIs) into autolysosomes. Although ATTECs are currently dominated by small molecules, the poor cell-type specificity and pharmacokinetic profile limit their applications in certain diseases. Moreover, the suboptimal intrinsic autophagic activity of cells affects the ATTECs-mediated degradation capability. Here we develop a nano-ATTEC system using our unique mixed-shell polymeric micelle (MSPM)-based nanoplatform for tumor-specific degradation of POIs. We demonstrate that the MSPMs-based nano-ATTEC is efficiently taken up by tumor cells in the acidic tumor microenvironment and to degrade POIs, rather than by normal cells under physiological conditions. More importantly, we find that this nano-ATTEC can not only target autolysosomes but also robustly enhance the autophagy activity, thereby establishing a positive feedback mechanism based on the autophagy pathway for efficient degradation of POIs. We believe that this MSPMs-based nano-ATTEC will find broad applications in tumor therapy.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.40
自引率
3.40%
发文量
209
审稿时长
1 months
期刊介绍:
ACS Macro Letters publishes research in all areas of contemporary soft matter science in which macromolecules play a key role, including nanotechnology, self-assembly, supramolecular chemistry, biomaterials, energy generation and storage, and renewable/sustainable materials. Submissions to ACS Macro Letters should justify clearly the rapid disclosure of the key elements of the study. The scope of the journal includes high-impact research of broad interest in all areas of polymer science and engineering, including cross-disciplinary research that interfaces with polymer science.
With the launch of ACS Macro Letters, all Communications that were formerly published in Macromolecules and Biomacromolecules will be published as Letters in ACS Macro Letters.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


