Toward selective electrooxidation of HMF to FDCA: Suppressing non-Faradaic transformations via low temperature electrolysis
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
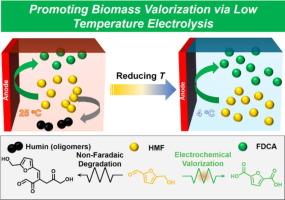
Electrooxidation of 5-hydroxymethylfurfural (HMF) to 2,5-furandicarboxylic acid (FDCA) is a promising strategy for biomass valorization. However, a significant challenge arises from the substantial carbon loss due to spontaneous HMF degradation in alkaline electrolytes, particularly at high concentrations. In this study, we present a straightforward approach to mitigating carbon loss during the electrochemical conversion of HMF to FDCA by suppressing non-Faradaic degradation through low-temperature electrolysis. Notably, under conventional room temperature conditions, carbon losses of up to 25 % can occur, whereas our low-temperature electrolysis method reduces carbon loss to negligible levels (< 1 %) even at high HMF concentrations (up to 500 mM) in highly alkaline electrolyte (2.0 M KOH). This strategy effectively addresses the long-standing challenge of balancing the enhanced kinetics of HMF electrooxidation with the accelerated degradation of HMF as its concentration increases in alkaline media. Our findings highlight the critical role of suppressing non-Faradaic degradation in the efficient conversion of HMF and demonstrate that low-temperature electrolysis offers a viable solution to the challenges of industrial-scale electrochemical biomass valorization.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


