Substitution–Leaching–Deposition (SLD) Processes Drive Reversible Surface Layer Reconstruction of Metal Oxides for Fluoride Adsorption
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
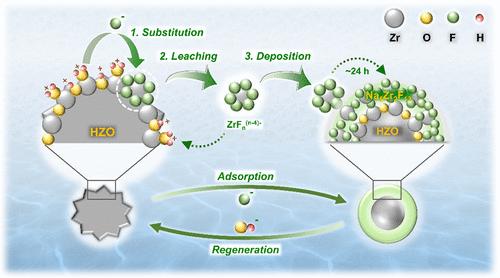
Surface complexation has long been recognized as the basic mode involved in fluoride adsorption onto metal oxides. However, such general recognition is challenged by the unusual pH dependence observed in fluoride adsorption. Here, we selected hydrated zirconium oxide (HZO) as a representative metal oxide to revisit the fluoride adsorption mechanism. Multiple in situ microscopic analyses and thermodynamic simulations suggest that, unlike the adsorption of other anions that proceed exclusively via substituting protonated terminal hydroxyl (η-OH2+) groups of metal oxides, fluoride can displace both η-OH2+ and protonated bridging hydroxyl (μ-OH+) groups of HZO (i.e., Substitution). This distinctive displacement drives the leaching of Zr from HZO, generating aqueous polyfluorozirconium complexes (i.e., Leaching) which subsequently deposit onto HZO via outer-sphere complexation (i.e., Deposition). The adsorbed polyfluorozirconium gradually converts into a fluorozirconate (Na5Zr2F13) coating, resulting in a surface layer reconstruction of up to 100 nm in depth. The atypical pH dependency of fluoride adsorption can be explained by the processes of Substitution, Leaching, and Deposition (i.e., SLD processes). More attractively, the SLD-driven surface layer reconstruction is reversible in nature, ensuring the constant defluoridation capability of HZO during cyclic adsorption-desorption assays. This study advances our understanding of fluoride adsorption at water-metal oxide interfaces.
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


