A polyketide-based biosynthetic platform for diols, amino alcohols and hydroxy acids
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Medium- and branched-chain diols and amino alcohols are important industrial solvents, polymer building blocks, cosmetics and pharmaceutical ingredients, yet biosynthetically challenging to produce. Here we present an approach that uses a modular polyketide synthase (PKS) platform for the efficient production of these compounds. This platform takes advantage of a versatile loading module from the rimocidin PKS and nicotinamide adenine dinucleotide phosphate-dependent terminal thioreductases. Reduction of the terminal aldehyde with alcohol dehydrogenases enables the production of diols, oxidation enables the production of hydroxy acids and specific transaminases allow the production of various amino alcohols. Furthermore, replacement of the malonyl-coenzyme A-specific acyltransferase in the extension module with methyl- or ethylmalonyl-coenzyme A-specific acyltransferase enables the production of branched-chain diols, amino alcohols and carboxylic acids in high titres. Use of our PKS platform in Streptomyces albus demonstrated the high tunability and efficiency of the platform. Medium- and branched-chain diols and amino alcohols are important industrial feedstocks, but they are biosynthetically challenging to produce. Here the authors introduce a modular polyketide synthase platform for the efficient production of these compounds.

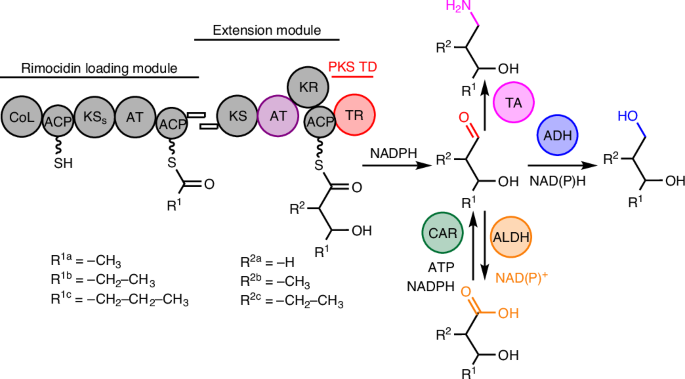
二醇、氨基醇和羟基酸的聚酮基生物合成平台
中链和支链二元醇和氨基醇是重要的工业溶剂、聚合物构件、化妆品和药物成分,但在生物合成方面却很难生产。在这里,我们介绍一种利用模块化聚酮合成酶(PKS)平台高效生产这些化合物的方法。该平台利用了来自rimocidin PKS 和烟酰胺腺嘌呤二核苷酸磷酸依赖性末端硫代还原酶的多功能装载模块。用醇脱氢酶还原末端醛可以生成二元醇,氧化可以生成羟基酸,特定的转氨酶可以生成各种氨基醇。此外,用甲基或乙基丙二酰辅酶 A-特异性酰基转移酶取代延伸模块中的丙二酰辅酶 A-特异性酰基转移酶,可以生产高滴度的支链二元醇、氨基醇和羧酸。在白链霉菌(Streptomyces albus)中使用我们的 PKS 平台证明了该平台的高可调性和高效性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


