A phase IIb randomised-controlled trial of the FFAR1/FFAR4 agonist icosabutate in MASH
IF 26.8
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background
Via regulation of glycemic control and inflammation, free fatty receptor (FFAR)1 and 4 are potential targets for the treatment of MASH. This study tested the efficacy and safety of icosabutate, a FFAR1/FFAR4 agonist, in MASH patients.Methods
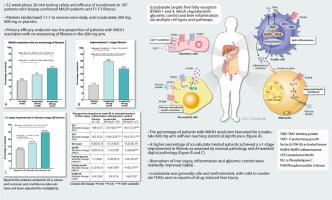
We performed a phase 2b, multicenter, 52-week, randomised, placebo-controlled trial (ICONA) testing the efficacy of icosabutate in MASH patients with F1-F3 (mild to severe) fibrosis. Patients were randomized 1:1:1 to receive once-daily, oral icosabutate 300 mg, 600 mg or placebo for 52 weeks. The primary efficacy endpoint was the proportion of patients with MASH resolution with no worsening of fibrosis in the 600 mg arm.Results
The primary population for efficacy analysis comprised 187 patients [placebo (n=62), 300 mg icosabutate (n= 58) or 600 mg icosabutate (n=67)]. The percentage of patients with MASH resolution favoured the icosabutate 600 mg arm without reaching statistical significance (23.9% vs. 14.5%; odds ratio, 2.01; 95% confidence interval [CI], 0.8 to 5.08; P=0.13). A higher percentage of patients treated with icosabutate achieved a ≥1-stage improvement in fibrosis, with a response rate of 29.3% in the 300 mg arm (odds ratio, 2.89; 95% CI, 1.09 to 7.70))and 23.9% in the 600 mg arm (odds ratio, 2.4; 95% CI, 0.90 to 6.37)) vs. 11.3% in the placebo arm. An improvement in fibrosis was observed using AI-assisted digital pathology. Marked decreases in biomarkers of liver damage were observed. Icosabutate was generally safe and well tolerated, with mild to moderate TEAEs and no reports of drug induced liver injury.Conclusion
Although the primary endpoint was not met, treatment with icosabutate demonstrated encouraging fibrosis (as measured by both conventional and AI-assisted digital pathology) and non-invasive biomarker data, supporting further development in MASH patients.ClinicalTrials.gov identifier
NCT04052516Impact and Implications
• With expression on multiple cells types regulating both glycemic control and liver inflammation, targeting free fatty acid receptors 1 and 4 could offer an attractive approach for the treatment of both fibrosing MASH and it’s comorbidities.• Although treatment of F1-F3 MASH patients with oral icosabutate (a FFAR1/FFAR4 agonist) did not meet the pre-defined primary endpoint (MASH resolution without worsening of fibrosis), the overall dataset (including AI-assisted digital pathology) suggest an improvement in fibrosis in treated patients.• Improvements in multiple biomarkers of liver damage, inflammation and glycemic control were observed in response to therapy.• Icosabutate was generally safe and well-tolerated, and the overall data support further testing of icosabutate in MASH patients, in particular those with more advanced disease (F2-F3 fibrosis) and type 2 diabetes.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hepatology
医学-胃肠肝病学
CiteScore
46.10
自引率
4.30%
发文量
2325
审稿时长
30 days
期刊介绍:
The Journal of Hepatology is the official publication of the European Association for the Study of the Liver (EASL). It is dedicated to presenting clinical and basic research in the field of hepatology through original papers, reviews, case reports, and letters to the Editor. The Journal is published in English and may consider supplements that pass an editorial review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


