Distinct mismatch-repair complex genes set neuronal CAG-repeat expansion rate to drive selective pathogenesis in HD mice
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
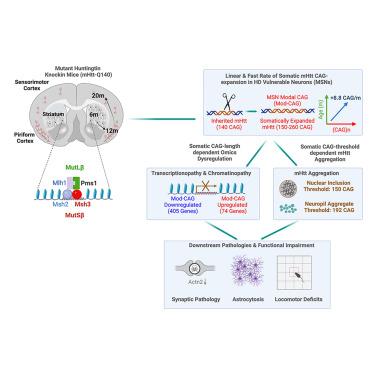
Huntington’s disease (HD) modifiers include mismatch-repair (MMR) genes, but their connections to neuronal pathogenesis remain unclear. Here, we genetically tested 9 HD genome-wide association study (GWAS)/MMR genes in mutant Huntingtin (mHtt) mice with 140 inherited CAG repeats (Q140). Knockout (KO) of genes encoding a distinct MMR complex either strongly (Msh3 and Pms1) or moderately (Msh2 and Mlh1) rescues phenotypes with early onset in striatal medium-spiny neurons (MSNs) and late onset in the cortical neurons: somatic CAG-repeat expansion, transcriptionopathy, and mHtt aggregation. Msh3 deficiency ameliorates open-chromatin dysregulation in Q140 neurons. Mechanistically, the fast linear rate of mHtt modal-CAG-repeat expansion in MSNs (8.8 repeats/month) is drastically reduced or stopped by MMR mutants. Msh3 or Pms1 deficiency prevents mHtt aggregation by keeping somatic MSN CAG length below 150. Importantly, Msh3 deficiency corrects synaptic, astrocytic, and locomotor defects in HD mice. Thus, Msh3 and Pms1 drive fast somatic mHtt CAG-expansion rates in HD-vulnerable neurons to elicit repeat-length/threshold-dependent, selective, and progressive pathogenesis in vivo.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


