Extracellular vesicles from the lung pro-thrombotic niche drive cancer-associated thrombosis and metastasis via integrin beta 2
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
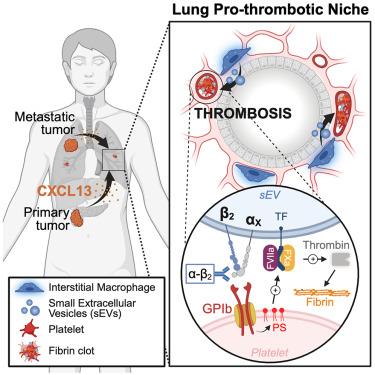
Cancer is a systemic disease with complications beyond the primary tumor site. Among them, thrombosis is the second leading cause of death in patients with certain cancers (e.g., pancreatic ductal adenocarcinoma [PDAC]) and advanced-stage disease. Here, we demonstrate that pro-thrombotic small extracellular vesicles (sEVs) are secreted by C-X-C motif chemokine 13 (CXCL13)-reprogrammed interstitial macrophages in the non-metastatic lung microenvironment of multiple cancers, a niche that we define as the pro-thrombotic niche (PTN). These sEVs package clustered integrin β2 that dimerizes with integrin αX and interacts with platelet-bound glycoprotein (GP)Ib to induce platelet aggregation. Blocking integrin β2 decreases both sEV-induced thrombosis and lung metastasis. Importantly, sEV-β2 levels are elevated in the plasma of PDAC patients prior to thrombotic events compared with patients with no history of thrombosis. We show that lung PTN establishment is a systemic consequence of cancer progression and identify sEV-β2 as a prognostic biomarker of thrombosis risk as well as a target to prevent thrombosis and metastasis.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


