Sol–Gel Synthesis of Phosphorylcholine Zwitterion-Decorated Silica Gels
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
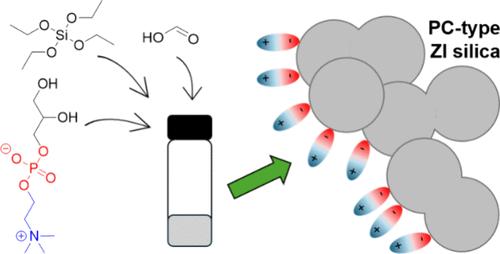
Zwitterion-functionalized silica particles are desirable as antifouling, highly hydrated, biocompatible materials. Existing methods to covalently attach zwitterionic groups to the silica particle surface generally require significant synthesis and purification procedures, and these have largely tended to focus on sulfobetaine-type zwitterionic moieties. This work describes a simple, one-pot, acid-catalyzed sol–gel synthesis approach to create phosphorylcholine (PC)-type zwitterionic silica gels via the condensation of hydroxyl groups on L-α-glycerophosphorylcholine (GPC) with silanol groups generated during the sol–gel reaction. The approach was successfully employed to create both PC-modified xerogels and ionogels (ionic liquid electrolyte-rich silica-supported gels). Silica gel particle morphologies and surfaces were characterized using scanning electron microscopy (SEM) with energy dispersive X-ray spectroscopy (EDS). EDS data revealed the presence of approximately 2–3 wt % phosphorus (from GPC) on all silica surfaces after thoroughly washing them postreaction. PC-functionalized ionogels displayed shear-thinning behavior and an approximately 2 to 4-fold increase in shear viscosity versus the control ionogel synthesized without GPC, while thermal analysis indicated that all ionogels yielded similar total silica content (5–7 wt %). This study indicates the promise of a simple, one-pot method for generating PC-decorated silica gels and presents future design possibilities for other novel materials leveraging zwitterionic molecules that possess hydroxyl groups.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


