Early-age efferocytosis directs macrophage arachidonic acid metabolism for tissue regeneration
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
In response to organ injury in adults, macrophages often promote scarring, yet during early life, they are required for tissue regeneration. To elucidate the mechanisms underlying age-associated regeneration, we compared the macrophage injury response in newborn versus adult hearts. Single-cell analysis revealed an accumulation of tissue-resident macrophages in neonates that were selectively polarized for apoptotic cell recognition and uptake (efferocytosis). Ablation of the apoptotic cell recognition receptor Mertk in newborns prevented cardiac regeneration. These findings could be attributed to reprogramming of macrophage gene expression that was required for biosynthesis of the eicosanoid thromboxane A2, which unexpectedly activated parenchymal cell proliferation. Markers of thromboxane A2 production were suppressed in adult macrophages after efferocytosis. Moreover, macrophage-neighboring neonatal cardiomyocytes expressed the thromboxane A2 receptor, whose activation induced a metabolic shift that supported cellular proliferation. Our data reveal a fundamental age-defined macrophage response in which lipid mitogens produced during efferocytosis support receptor-mediated tissue regeneration.
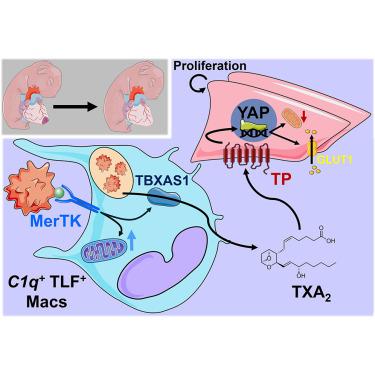
早期efferocytosis指导巨噬细胞花生四烯酸代谢组织再生
在成人器官损伤的反应中,巨噬细胞经常促进瘢痕形成,但在生命早期,它们是组织再生所必需的。为了阐明年龄相关再生的机制,我们比较了新生儿和成人心脏巨噬细胞损伤反应。单细胞分析揭示了新生儿组织内巨噬细胞的积累,这些巨噬细胞被选择性极化以识别和摄取凋亡细胞(efferocytosis)。新生儿凋亡细胞识别受体Mertk的消融可阻止心脏再生。这些发现可能归因于巨噬细胞基因表达的重编程,这是生物合成二十烷类血栓素A2所必需的,它意外地激活了实质细胞的增殖。成年巨噬细胞在efferocytosis后,血栓素A2的生成标记物被抑制。此外,巨噬细胞附近的新生儿心肌细胞表达血栓素A2受体,其激活诱导了支持细胞增殖的代谢转变。我们的数据揭示了一个基本的年龄定义的巨噬细胞反应,其中在efferocytosis过程中产生的脂质有丝分裂原支持受体介导的组织再生。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


