Structural Reconstruction via Carbon Nanotube Spatially Confined Metal Catalysis: A Morphology-Controlled Approach to Convert Polycyclic Aromatic Hydrocarbon into Carbon Nanofibers for Highly Active Anodes in Li-Ion Batteries
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
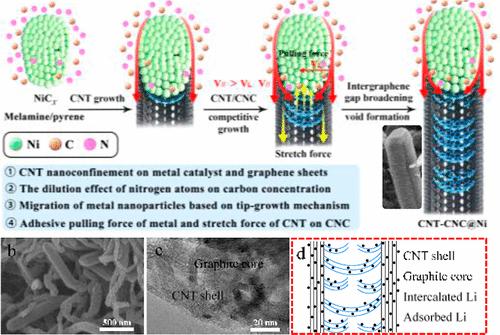
By a carbon nanotube (CNT) spatially confined metal-catalyzed structural reconstruction, carbon nanofibers (CNFs) with a hollow, hollow-solid, solid graphite core, and CNT shell are prepared using nitrogen heterocycle (NHC) and polycyclic aromatic hydrocarbon (PAH) as carbon sources. The formation mechanism of CNFs with oriented graphene layers and enlarged intergraphene spacing is studied by X-ray diffraction, scanning electron microscopy, transmission electron microscopy, and selected area electron diffraction analysis. It revealed that this one-dimensional nanoconfined metal-catalyzed carbon rearrangement is totally different from the reported spatially localized metal-catalyzed graphitization of electrospun polymer and nanocasted carbohydrate nanofibers, as the graphene orientation, cavity volume, and interlayer distance of CNFs can be controlled by the carbon concentration-related competitive metal-catalyzed tip growth of latitudinal and longitudinal graphene layers from NHC and PAH. The unique CNF structure renders good electronic/ionic conductivity, abundant Li+ storage interlayer gaps, and robust mechanical durability, resulting in outstanding electrochemical properties as anodes in lithium-ion batteries. The optimum CNF anode delivers a stable discharge capacity of 475 mA h g–1 at 0.1 C, an extraordinary rate capability of 303 mA h g–1 at 5 C, and a remarkable long-term cycling stability of 378 mA h g-1 after 600 cycles at 1 C. This 1D nanoconfined metal catalysis synthesis could be useful for the development of efficient CNF anodes in many electrochemical reactions with a potential for industrial applications.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


