Absence of Long-Range Magnetic Ordering in a Trirutile High-Entropy Oxide (Mn0.2Fe0.2Co0.2Ni0.2Cu0.2)Ta1.92O6−δ
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
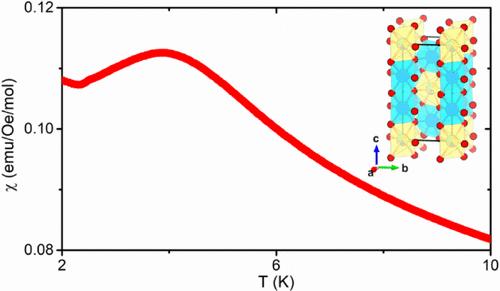
Functionalities of solid-state materials are usually considered to be dependent on their crystal structures. The limited structural types observed in the emerging high-entropy oxides put constraints on the exploration of their physical properties and potential applications. Herein, we synthesized the first high-entropy oxide in a trirutile structure, (Mn0.2Fe0.2Co0.2Ni0.2Cu0.2)Ta1.92O6−δ, and investigated its magnetism. The phase purity and high-entropy nature were confirmed by powder X-ray diffraction and energy-dispersive spectroscopy, respectively. X-ray photoelectron spectroscopy indicated divalent Mn, Co, Ni, and Cu along with trivalent Fe. Magnetic property measurements showed antiferromagnetic coupling and potential short-range magnetic ordering below ∼4 K. The temperature-dependent heat capacity data measured under zero and high magnetic fields confirmed the lack of long-range magnetic ordering and a possible low-temperature phonon excitation. The discovery of the first trirutile high-entropy oxide opens a new pathway for studying the relationship between the highly disordered atomic arrangement and magnetic interaction. Furthermore, it provides a new direction for exploring the functionalities of high-entropy oxides.
三元高熵氧化物(Mn0.2Fe0.2Co0.2Ni0.2Cu0.2) ta1.92 2o6−δ中远程磁有序的缺失
固态材料的功能通常被认为依赖于它们的晶体结构。在新出现的高熵氧化物中观察到的有限结构类型限制了其物理性质和潜在应用的探索。本文合成了第一种三元结构的高熵氧化物(Mn0.2Fe0.2Co0.2Ni0.2Cu0.2) ta1.92 2o6−δ,并对其磁性进行了研究。通过粉末x射线衍射和能量色散光谱分别证实了其相纯度和高熵性质。x射线光电子能谱显示二价Mn、Co、Ni、Cu和三价Fe。磁性能测量显示反铁磁耦合和在~ 4 K以下的潜在短程磁有序。在零磁场和高磁场下测量的温度相关热容数据证实了缺乏长程磁有序和可能的低温声子激发。第一种三维高熵氧化物的发现,为研究高度无序的原子排列与磁相互作用之间的关系开辟了新的途径。这也为高熵氧化物的官能团研究提供了新的方向。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


