Rapid Bacterial Identification through Multiplexed Nucleic Acid Detection on a Digital Microfluidic Platform for Enhanced Clinical Intervention against Infections
IF 9.1
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Bacterial infections often lead to severe health consequences owing to their ability to infiltrate multiple anatomical sites, including the bloodstream, respiratory tract, and digestive tract, posing substantial diagnostic and therapeutic challenges. Consequently, a rapid and versatile detection method capable of identifying a broad spectrum of bacterial pathogens is urgently required to facilitate precise antibiotic prescriptions. Addressing this need, we introduce MiND-DMF (Multibacterial Infection Nucleic Acid Detection on a Digital Microfluidic Platform), a cost-effective digital microfluidic platform tailored for multiplexed bacterial detection. This system integrates DNA extraction, recombinase polymerase amplification (RPA), and CRISPR-based detection technologies, enabling the efficient identification of six common infectious bacteria. Operating at a constant temperature of 37 °C, MiND-DMF completes the entire diagnostic process in just 55 min and is compatible with human reference genes. In spiked samples, the platform demonstrated a detection limit of 100 CFU/mL, highlighting its exceptional sensitivity and quantification capability. In clinical evaluations, MiND-DMF exhibited outstanding performance, achieving 100% sensitivity and 98%–100% specificity compared to conventional PCR methods across 50 samples derived from diverse tissue sources. This robust platform demonstrates strong anti-interference capabilities, making it suitable for analyzing various tissue fluids including blood, alveolar lavage fluid, urine, nasal secretions, appendiceal pus, and ear pus. The versatility and precision of MiND-DMF support the monitoring of hospital-acquired bacterial infection origins, empowering physicians to prescribe targeted antibiotics and enhancing overall infection prevention and control strategies. By accurately detecting bacteria from multiple sources, MiND-DMF can play a pivotal role in improving patient outcomes and public health.
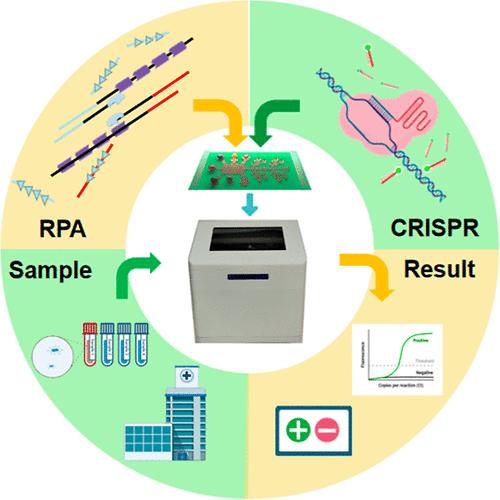
基于数字微流控平台的多路核酸检测快速细菌鉴定增强临床感染干预
细菌感染往往会导致严重的健康后果,因为它们能够渗透到多个解剖部位,包括血液、呼吸道和消化道,给诊断和治疗带来了重大挑战。因此,迫切需要一种能够识别广谱细菌病原体的快速和通用的检测方法,以促进精确的抗生素处方。为了满足这一需求,我们推出了MiND-DMF(数字微流控平台上的多细菌感染核酸检测),这是一种为多路细菌检测量身定制的具有成本效益的数字微流控平台。该系统集成了DNA提取、重组酶聚合酶扩增(RPA)和基于crispr的检测技术,能够有效识别六种常见的感染性细菌。MiND-DMF在37°C的恒定温度下工作,只需55分钟即可完成整个诊断过程,并与人类参考基因兼容。在加标样品中,该平台的检测限为100 CFU/mL,突出了其卓越的灵敏度和定量能力。在临床评估中,MiND-DMF表现出出色的性能,与传统PCR方法相比,在来自不同组织来源的50个样本中实现了100%的灵敏度和98%-100%的特异性。该平台具有较强的抗干扰能力,适用于分析血液、肺泡灌洗液、尿液、鼻分泌物、阑尾脓液、耳脓液等多种组织液体。MiND-DMF的多功能性和精确性支持对医院获得性细菌感染源头的监测,使医生能够开出有针对性的抗生素,并加强整体感染预防和控制策略。MiND-DMF通过准确检测多种来源的细菌,可以在改善患者预后和公共卫生方面发挥关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sensors
Chemical Engineering-Bioengineering
CiteScore
14.50
自引率
3.40%
发文量
372
期刊介绍:
ACS Sensors is a peer-reviewed research journal that focuses on the dissemination of new and original knowledge in the field of sensor science, particularly those that selectively sense chemical or biological species or processes. The journal covers a broad range of topics, including but not limited to biosensors, chemical sensors, gas sensors, intracellular sensors, single molecule sensors, cell chips, and microfluidic devices. It aims to publish articles that address conceptual advances in sensing technology applicable to various types of analytes or application papers that report on the use of existing sensing concepts in new ways or for new analytes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


