Igniting the catalytic activity of Mn-Fe spinel for Fenton reactions: The doped nitrogen atoms in the interstitial sites and substitutional sites
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
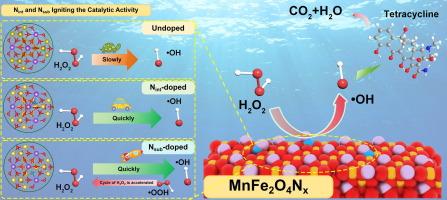
Nitrogen doping plays a crucial role in activating the Fenton catalytic activity of transition metal oxides. However, the mechanism by which nitrogen doping sites influence the properties of these oxides remains unclear. We successfully incorporated nitrogen atoms into the internal lattice of MnFe2O4 spinel through thermal activation and identified two major doping sites: interstitial nitrogen (Nint) and substitutional nitrogen (Nsub). Through in-situ DIRFTS experiments and DFT calculations, we proposed a unique mechanism. Both sites can effectively activate the catalytic activity of the oxides, but Nsub is particularly effective in altering the electron distribution of Mn elements within the lattice. This alteration promotes the transformation of superoxide (•O2–/•OOH) to H2O2, thereby further enhancing catalytic activity. Compared to un-doped MnFe2O4, experimental results obtained using MnFe2O4N0.4 as the catalyst demonstrated that the degradation rate constant of tetracycline in simulated wastewater increased from 0.0072 min−1 to 0.2745 min−1. Furthermore, the degradation rate of tetracycline reached 98.7 %. For real wastewater experiments, the COD removal rate was 77.4 %, and the mineralization rate was 62.5 %. This study offers new insights into the design of nitrogen-doped transition metal oxide catalysts.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


