Synergy-Promoted Specific Alkyltriphenylphosphonium Binding to CB[8]
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Biological substrate specificity ensures that organisms interact accurately with biomolecular receptors, crucial for key functions such as signaling and immunity. Nevertheless, this phenomenon is still poorly understood, with host–guest chemistry offering a suitable platform for studying simplified models. Herein, we report an in-depth study of the host–guest chemistry of alkyltriphenylphosphonium cations with cucurbit[8]uril (CB[8]), initiated by the serendipitous discovery of salt forming a tightly bound pseudoheteroternary 1:1 complex with CB[8]. A first generation of model substrates was designed to explore an unusual binding mode characterized by the simultaneous introduction of two distinct guest fragments within the host cavity. Structural features of the complexes were elucidated using ESI-MS and NMR 1D/2D techniques; thermodynamic properties were assessed by isothermal titration calorimetry, and kinetic parameters were derived from selective inversion–recovery NMR. Experimental results aligned well with electronic structure calculations, revealing a reproducible binding motif with submicromolar affinities. This peculiar complexation mode involves a synergistic effect caused by steric crowding around the P+ atom, facilitating the insertion of two aromatic units into CB[8] while hindering association with CB[7]. Based on these findings, a second generation of minimalistic substrates was developed, preserving the synergistic interaction mode and exhibiting specific binding to CB[8].
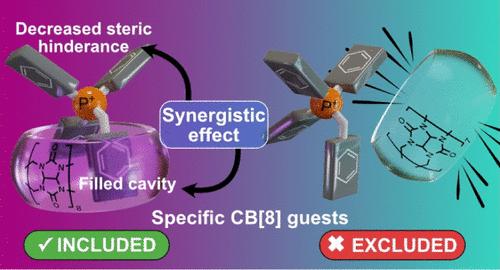
协同促进特异性烷基三苯磷与cb[8]的结合
生物底物特异性确保生物体与生物分子受体准确相互作用,对信号和免疫等关键功能至关重要。然而,这种现象仍然知之甚少,主客体化学为研究简化模型提供了一个合适的平台。在此,我们报道了一项深入研究了烷基三苯基磷阳离子与葫芦bb[8] (CB[8])的主客体化学,由偶然发现的盐与CB[8]形成紧密结合的假杂三元1:1配合物引发。第一代模型基板旨在探索一种不同寻常的结合模式,其特征是在宿主腔内同时引入两个不同的客体片段。利用ESI-MS和NMR 1D/2D技术对配合物的结构特征进行了表征;热力学性质由等温滴定量热法评估,动力学参数由选择性反演-恢复核磁共振获得。实验结果与电子结构计算结果一致,揭示了具有亚微摩尔亲和力的可重复结合基序。这种特殊的络合模式涉及到由P+原子周围的空间拥挤引起的协同效应,促进了两个芳香单元插入到CB[8]中,同时阻碍了与CB[7]的结合。基于这些发现,开发了第二代极简底物,保留了协同相互作用模式,并表现出与CB[8]的特异性结合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


