Harnessing Amino Acid Modularity for Programmable Function in Covalent Peptide Assemblies
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
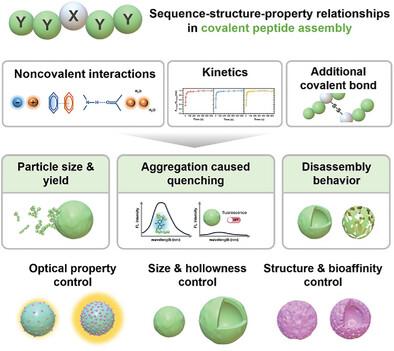
Covalent peptide assembly leverages robust covalent bonds and dynamic non-covalent interactions to provide enhanced stability and introduce diverse functionalities. Nevertheless, it remains significantly challenging to achieve modular control over the structural diversity and functional complexity while elucidating how specific amino acid sequences contribute to these processes. Here, the systematic encoding of peptide derivative characteristics is demonstrated through amino acid modularity to enable precise control over both the structural diversity and functional complexity in covalent peptide assemblies. By systematically screening single amino acid substitutions in pentapeptides using tyrosine crosslinking, a diverse library of peptide constructs is developed. Each construct is tailored to exhibit distinct properties, including charge repulsion, aggregation-induced quenching, disassembly behavior, and redox responsiveness. The strategic manipulation of sequence composition, both in individual assemblies and combinatorial systems, enables programmable control over the structural diversity and functional complexity. This approach yields various module-specific functions, including frustrated growth, hierarchical hollow architecture formation, affinity enrichment, stimuli-responsive behavior, and fluorescence signal amplification. This work establishes a framework for the design of modular peptide materials with programmable functionalities, advancing the development of next-generation multicomponent peptide assembly technologies characterized by unprecedented complexity and adaptability.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


