Carbonation of MgO Single Crystals: Implications for Direct Air Capture of CO2
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
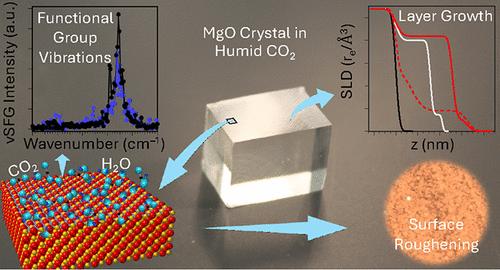
Direct air capture (DAC) may be feasible to remove carbon dioxide (CO2) from the atmosphere at the gigaton scale, holding promise to become a major contributor to climate change mitigation. Mineral looping using magnesium oxide (MgO) is potentially an economical, efficient, and sustainable pathway to gigaton-scale DAC. The hydroxylation and carbonation of MgO determine the efficiency of the looping process, but their rates and mechanisms remain uncertain. In this work, MgO single crystals were reacted in air or CO2 at varying humidities and characterized by X-ray scattering, microscopy, and vibrational spectroscopy. Results show that the hydroxylation formed a brucite (Mg(OH)2)-like layer immediately after crystal cleaving. Concurrently, the carbonation formed hydrated magnesium carbonate phases, including barringtonite (MgCO3·2H2O) and nesquehonite (MgCO3·2H2O), in the layer. Rapid initial growth of the layer is also manifested in short-range bending/warping of nanocrystallites, resulting in multiple orientations of the same phases on the surface. The layer growth slowed down over time, indicating surface passivation. The formation of barringtonite and nesquehonite with 1:1 CO3/Mg ratio indicates an efficient carbonation when compared to other magnesium carbonate phases of lower ratio. Our results are essential for understanding surface passivation mechanisms and tackling the passivation issue of mineral looping DAC technology.
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


